Effects of low-dose oxygen ions and protons on cardiac function and structure in male C57BL/6J mice
- PMID: 30797436
- PMCID: PMC6391741
- DOI: 10.1016/j.lssr.2019.01.003
Effects of low-dose oxygen ions and protons on cardiac function and structure in male C57BL/6J mice
Abstract
Purpose: Astronauts traveling beyond low-Earth orbit will be exposed to high linear-energy transfer charged particles. Because there is concern about the adverse effects of space radiation on the cardiovascular system, this study assessed cardiac function and structure and immune cell infiltration in a mouse model of charged-particle irradiation.
Materials and methods: Male C57BL/6 J mice were exposed to oxygen ions (16O, 600 MeV/n at 0.25-0.26 Gy/min to a total dose of 0, 0.05, 0.1, 0.25, or 1 Gy), protons (150 MeV, 0.35-0.55 Gy/min to 0, 0.5, or 1 Gy), or protons (150 MeV, 0.5 Gy) followed by 16O (600 MeV/n, 0.1 Gy). Separate groups of mice received 137Cs γ-rays (1 Gy/min to 0, 0.5, 1, or 3 Gy) as a reference. Cardiac function and blood velocity were measured with ultrasonography at 3, 5, 7, and 9 months after irradiation. At 2 weeks, 3 months, and 9 months, cardiac tissue was collected to assess apoptosis, tissue remodeling, and markers of immune cells.
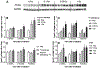
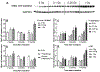
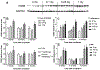
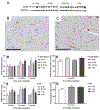
Results: Ejection fraction and fractional shortening decreased at 3 and 7 months after 16O. These parameters did not change in mice exposed to γ-rays, protons, or protons followed by 16O. Each of the radiation exposures caused only small increases in cleaved caspase-3 and numbers of apoptotic nuclei. Changes in the levels of α-smooth muscle cell actin and a 75-kDa peptide of collagen type III in the left ventricle suggested tissue remodeling, but there was no significant change in total collagen deposition at 2 weeks, 3 months, and 9 months. Increases in protein amounts of cluster of differentiation (CD)2, CD68, and CD45 as measured with immunoblots at 2 weeks, 3 months, and 9 months after exposure to protons or 16O alone suggested immune cell infiltration. For type III collagen, CD2 and CD68, the efficacy in inducing protein abundance of CD2, CD68, and CD45 was 16O > protons > γ-rays > protons followed by 16O.
Conclusions: Low-dose, high-energy charged-particle irradiation caused mild changes in cardiac function and tissue remodeling in the mouse.
Keywords: Cardiovascular system; Degenerative tissue effects; Immune cell infiltration; Mouse model; Space radiation.
Copyright © 2019 The Committee on Space Research (COSPAR). Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure statement
The authors report no conflicts of interest.
Figures

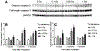
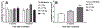
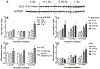
References
-
- Cucinotta FA and Durante M. Cancer risk from exposure to galactic cosmic rays: implications for space exploration by human beings. Lancet Oncol 2006; 7:431–435. - PubMed
-
- Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol 2003; 45:55–75. - PubMed
-
- Heidenreich PA, Hancock SL, Vagelos RH, Lee BK, Schnittger I. Diastolic dysfunction after mediastinal irradiation. Am Heart J 2005; 150:977–982. - PubMed
-
- Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res 2003; 160:381–407. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

