Differential durability of immune responses to measles and mumps following MMR vaccination
- PMID: 30797639
- PMCID: PMC6414234
- DOI: 10.1016/j.vaccine.2019.02.030
Differential durability of immune responses to measles and mumps following MMR vaccination
Abstract
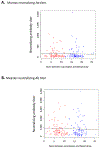
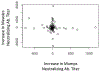
The development and wide-spread use of mumps vaccine resulted in a dramatic and sustained decrease in the incidence of mumps disease; however, since 2000, an increase in the size and number of mumps outbreaks in the United States and other countries has sparked renewed interest in the durability of mumps-specific immunity elicited by mumps vaccination. The most likely explanation for mumps cases in previously immunized persons may be secondary vaccine failure, or waning immunity. In the current study, we examined changes in markers of measles and mumps immunity at two timepoints, approximately 7 and 17 years after two-dose MMR-II® vaccination, in a cohort of 98 healthy adults. Our results indicate that mumps IgG titers exhibited a large and significant decline during this time period, while mumps neutralizing Ab titers were relatively stable. There was a similar discrepancy with measles-specific immune responses. For both pathogens, neutralizing antibody titers were fairly low and, given the length of time since vaccination, may have already declined. These data suggest that specific immune outcomes may wane at different rates and highlight our currently incomplete understanding of protective immune responses to mumps and measles.
Keywords: Antibodies; Cell-mediated immunity; Humoral immunity; MMR-II vaccine; Measles; Measles vaccine; Measles virus; Mumps; Mumps vaccine; Mumps virus; T cell ELISPOT.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosures
Dr. Poland is the chair of a Safety Evaluation Committee for novel investigational vaccine trials being conducted by Merck Research Laboratories. Dr. Poland offers consultative advice on vaccine development to Merck & Co. Inc., Avianax, Adjuvance, Valneva, Medicago, Sanofi Pasteur, GlaxoSmithKline, and Emergent Biosolutions. Dr. Poland’s effort on this project was covered by the abovementioned NIH grants. Dr. Poland did not receive any support from the Merck Investigator Studies Program grant. Drs. Poland and Ovsyannikova hold patents related to measles and vaccinia peptide research. Dr. Kennedy holds a patent related to vaccinia peptide research. Dr. Kennedy has received funding from the Merck Investigator Studies Program to study waning immunity to mumps vaccine. These activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic Conflict of Interest policies.
Dr. Poland is the chair of a Safety Evaluation Committee for novel non-rubella investigational vaccine trials being conducted by Merck Research Laboratories. Dr. Poland offers consultative advice on vaccine development to Merck & Co. Inc., Avianax, Adjuvance, Valneva, Medicago, Sanofi Pasteur, GlaxoSmithKline, and Emergent Biosolutions. Drs. Poland and Ovsyannikova hold three patents on measles and vaccinia peptide research. Dr. Kennedy holds a patent on vaccinia peptide research. Dr. Kennedy has also received funding from Merck Research Laboratories to study waning immunity to mumps. All other authors declare no competing financial interests. These activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic Conflict of Interest policies. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies.
Figures





References
-
- Rubin S Mumps Vaccines In: Plotkin S, Orenstein W, Offit P, Edwards KM, editors. Plotkin’s VACCINES. 7th ed. Philadelphia, PA: Elsevier, 2017: pp. 663–87.
-
- Rafiei Tabatabaei S, Esteghamati AR, Shiva F, Fallah F, Radmanesh R, Abdinia B, et al. Detection of serum antibodies against measles, mumps and rubella after primary measles, mumps and rubella (MMR) vaccination in children. Arch Iran Med 2013. Jan;16(1):38–41. - PubMed
-
- Weibel RE, Stokes J Jr., Buynak EB, Whitman JE Jr, Hilleman MR. Live, attenuated mumps-virus vaccine. 3. Clinical and serologic aspects in a field evaluation. New Engl J of Med 1967;276:245–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

