Physical Basis for the Loading of a Bacterial Replicative Helicase onto DNA
- PMID: 30797687
- PMCID: PMC6450724
- DOI: 10.1016/j.molcel.2019.01.023
Physical Basis for the Loading of a Bacterial Replicative Helicase onto DNA
Abstract
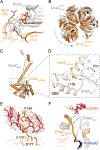
In cells, dedicated AAA+ ATPases deposit hexameric, ring-shaped helicases onto DNA to initiate chromosomal replication. To better understand the mechanisms by which helicase loading can occur, we used cryo-EM to determine sub-4-Å-resolution structures of the E. coli DnaB⋅DnaC helicase⋅loader complex with nucleotide in pre- and post-DNA engagement states. In the absence of DNA, six DnaC protomers latch onto and crack open a DnaB hexamer using an extended N-terminal domain, stabilizing this conformation through nucleotide-dependent ATPase interactions. Upon binding DNA, DnaC hydrolyzes ATP, allowing DnaB to isomerize into a topologically closed, pre-translocation state competent to bind primase. Our data show how DnaC opens the DnaB ring and represses the helicase prior to DNA binding and how DnaC ATPase activity is reciprocally regulated by DnaB and DNA. Comparative analyses reveal how the helicase loading mechanism of DnaC parallels and diverges from homologous AAA+ systems involved in DNA replication and transposition.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTEREST
The authors declare no competing interests.
Figures





Similar articles
-
Convergent evolution in two bacterial replicative helicase loaders.Trends Biochem Sci. 2022 Jul;47(7):620-630. doi: 10.1016/j.tibs.2022.02.005. Epub 2022 Mar 26. Trends Biochem Sci. 2022. PMID: 35351361 Free PMC article. Review.
-
The molecular coupling between substrate recognition and ATP turnover in a AAA+ hexameric helicase loader.Elife. 2021 May 26;10:e64232. doi: 10.7554/eLife.64232. Elife. 2021. PMID: 34036936 Free PMC article.
-
DnaC, the indispensable companion of DnaB helicase, controls the accessibility of DnaB helicase by primase.J Biol Chem. 2017 Dec 22;292(51):20871-20882. doi: 10.1074/jbc.M117.807644. Epub 2017 Oct 25. J Biol Chem. 2017. PMID: 29070678 Free PMC article.
-
Biochemical characterization of Escherichia coli DnaC variants that alter DnaB helicase loading onto DNA.J Biol Chem. 2024 May;300(5):107275. doi: 10.1016/j.jbc.2024.107275. Epub 2024 Apr 6. J Biol Chem. 2024. PMID: 38588814 Free PMC article.
-
Structural Insight Into the Function of DnaB Helicase in Bacterial DNA Replication.Proteins. 2025 Feb;93(2):420-429. doi: 10.1002/prot.26746. Epub 2024 Sep 4. Proteins. 2025. PMID: 39230358 Review.
Cited by
-
Embracing Heterogeneity: Challenging the Paradigm of Replisomes as Deterministic Machines.Chem Rev. 2023 Dec 13;123(23):13419-13440. doi: 10.1021/acs.chemrev.3c00436. Epub 2023 Nov 16. Chem Rev. 2023. PMID: 37971892 Free PMC article. Review.
-
Convergent evolution in two bacterial replicative helicase loaders.Trends Biochem Sci. 2022 Jul;47(7):620-630. doi: 10.1016/j.tibs.2022.02.005. Epub 2022 Mar 26. Trends Biochem Sci. 2022. PMID: 35351361 Free PMC article. Review.
-
Multiple roles of Pol epsilon in eukaryotic chromosome replication.Biochem Soc Trans. 2022 Feb 28;50(1):309-320. doi: 10.1042/BST20210082. Biochem Soc Trans. 2022. PMID: 35129614 Free PMC article.
-
The molecular coupling between substrate recognition and ATP turnover in a AAA+ hexameric helicase loader.Elife. 2021 May 26;10:e64232. doi: 10.7554/eLife.64232. Elife. 2021. PMID: 34036936 Free PMC article.
-
Structural Insights of the DciA Helicase Loader in Its Relationship with DNA.Int J Mol Sci. 2023 Jan 11;24(2):1427. doi: 10.3390/ijms24021427. Int J Mol Sci. 2023. PMID: 36674944 Free PMC article.
References
-
- Alley SC, Shier VK, Abel-Santos E, Sexton DJ, Soumillion P, and Benkovic SJ (1999). Sliding Clamp of the Bacteriophage T4 Polymerase Has Open and Closed Subunit Interfaces in Solution. Biochemistry 38, 7696–7709. - PubMed
-
- Alley SC, Abel-Santos E, and Benkovic SJ (2000). Tracking sliding clamp opening and closing during bacteriophage T4 DNA polymerase holoenzyme assembly. Biochemistry 39, 3076–3090. - PubMed
-
- Arai K, and Kornberg A (1981). Mechanism of dnaB protein action. II. ATP hydrolysis by dnaB protein dependent on single- or double-stranded DNA. J. Biol. Chem 256, 5253–5259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

