Pulmonary cytomegalovirus (CMV) DNA shedding in allogeneic hematopoietic stem cell transplant recipients: Implications for the diagnosis of CMV pneumonia
- PMID: 30797790
- PMCID: PMC7126576
- DOI: 10.1016/j.jinf.2019.02.009
Pulmonary cytomegalovirus (CMV) DNA shedding in allogeneic hematopoietic stem cell transplant recipients: Implications for the diagnosis of CMV pneumonia
Abstract
Objectives: To date no definitive cut-off value for cytomegalovirus (CMV) DNA load in bronchoalveolar lavage (BAL) fluid specimens has been established to discriminate between CMV pneumonia and pulmonary CMV DNA shedding in allogeneic hematopoietic stem cell transplant (allo-HSCT) recipients.
Methods: The current retrospective study is aimed at assessing the range of CMV DNA loads quantified in BAL fluid specimens from allo-HSCT patients with pneumonia in which different microorganisms were causally involved.
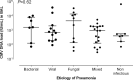
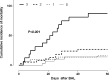
Results: A total of 144 BAL specimens from 123 patients were included. CMV DNA was detected in 56 out of 144 BAL fluid specimens and the median CMV DNA load from patients in whom CMV pneumonia was unlikely or could be tentatively ruled out was 1210 (31-68, 920) IU/ml. The frequency of CMV DNA detection and median CMV DNA loads were comparable, irrespective of the attributable cause of pneumonia. Detection of CMV DNA loads in BAL fluid specimens >500 IU/ml was independently associated with pneumonia-attributable mortality.
Conclusions: The current study highlights the difficulty in establishing universal CMV DNA load thresholds in BAL fluid specimens for distinguishing between CMV pneumonia and pulmonary CMV DNA shedding, and suggests that the presence of CMV DNA in BAL fluid specimens beyond a certain level may have a deleterious impact on patient outcome.
Keywords: CMV DNA in BAL; CMV DNAemia; CMV pneumonia; Cytomegalovirus; Pre-emptive antiviral therapy.
Copyright © 2019. Published by Elsevier Ltd.
Figures

Similar articles
-
Cytomegalovirus (CMV) DNA Quantitation in Bronchoalveolar Lavage Fluid From Hematopoietic Stem Cell Transplant Recipients With CMV Pneumonia.J Infect Dis. 2017 May 15;215(10):1514-1522. doi: 10.1093/infdis/jix048. J Infect Dis. 2017. PMID: 28181657 Free PMC article.
-
Cytomegalovirus (CMV) DNA quantification in bronchoalveolar lavage fluid of immunocompromised patients with CMV pneumonia.Clin Transplant. 2018 Jan;32(1). doi: 10.1111/ctr.13149. Epub 2017 Dec 8. Clin Transplant. 2018. PMID: 29112278
-
Monitoring of oral cytomegalovirus DNA shedding for the prediction of viral DNAemia in allogeneic hematopoietic stem cell transplant recipients.J Med Virol. 2018 Aug;90(8):1375-1382. doi: 10.1002/jmv.25185. Epub 2018 Apr 26. J Med Virol. 2018. PMID: 29663435
-
Evaluation of COBAS AmpliPrep/COBAS TaqMan CMV Test for use in hematopoietic stem cell transplant recipients.Expert Rev Mol Diagn. 2017 Jul;17(7):633-639. doi: 10.1080/14737159.2017.1325737. Epub 2017 May 15. Expert Rev Mol Diagn. 2017. PMID: 28468570 Review.
-
Assessment and prevention of cytomegalovirus infection in allogeneic hematopoietic stem cell transplant and in solid organ transplant: A multidisciplinary consensus conference by the Italian GITMO, SITO, and AMCLI societies.Clin Transplant. 2019 Oct;33(10):e13666. doi: 10.1111/ctr.13666. Epub 2019 Aug 6. Clin Transplant. 2019. PMID: 31310687 Review.
Cited by
-
The plasma viral communities associate with clinical profiles in a large-scale haematological patients cohort.Microbiome. 2024 Jul 23;12(1):137. doi: 10.1186/s40168-024-01855-4. Microbiome. 2024. PMID: 39044261 Free PMC article.
-
Delayed Onset of COVID-19 in an Immunosuppressed Patient.Infect Dis Clin Pract (Baltim Md). 2021 Nov;29(6):e448-e450. doi: 10.1097/IPC.0000000000001027. Epub 2021 May 15. Infect Dis Clin Pract (Baltim Md). 2021. PMID: 34803351 Free PMC article.
-
Performance evaluation of the Alinity m system for quantifying cytomegalovirus DNA in samples of the respiratory, gastrointestinal, and urinary tract.Microbiol Spectr. 2024 Jul 2;12(7):e0420123. doi: 10.1128/spectrum.04201-23. Epub 2024 Jun 6. Microbiol Spectr. 2024. PMID: 38842363 Free PMC article.
-
Uniform graft-versus-host disease prophylaxis with posttransplant cyclophosphamide, sirolimus, and mycophenolate mofetil following hematopoietic stem cell transplantation from haploidentical, matched sibling and unrelated donors.Bone Marrow Transplant. 2020 Nov;55(11):2147-2159. doi: 10.1038/s41409-020-0921-6. Epub 2020 May 5. Bone Marrow Transplant. 2020. PMID: 32371901
-
Cytomegalovirus (CMV) Pneumonitis: Cell Tropism, Inflammation, and Immunity.Int J Mol Sci. 2019 Aug 8;20(16):3865. doi: 10.3390/ijms20163865. Int J Mol Sci. 2019. PMID: 31398860 Free PMC article. Review.
References
-
- Pérez Romero P., Blanco P., Giménez E., Solano C., Navarro D. An update on the management and prevention of cytomegalovirus infection following allogeneic hematopoietic stem cell transplantation. Futur Virol. 2015;10:113–134.
-
- Ljungman P., Boeckh M., Hirsch H.H., Josephson F., Lundgren J., Nichols G., et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64:87–91. - PubMed
-
- Crawford S.W., Hackman R.C., Clark J.G. Open lung biopsy diagnosis of diffuse pulmonary infiltrates after marrow transplantation. Chest. 1988;94:949–953. - PubMed

