Neuroendocrinology of reproduction: Is gonadotropin-releasing hormone (GnRH) dispensable?
- PMID: 30797802
- PMCID: PMC7216701
- DOI: 10.1016/j.yfrne.2019.02.002
Neuroendocrinology of reproduction: Is gonadotropin-releasing hormone (GnRH) dispensable?
Abstract
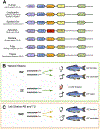
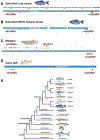
Gonadotropin releasing hormone (GnRH) is a highly conserved neuroendocrine decapeptide that is essential for the onset of puberty and the maintenance of the reproductive state. First identified in mammals, the GnRH signaling pathway is found in all classes of vertebrates; homologues of GnRH have also been identified in invertebrates. In addition to its role as a hypothalamic releasing hormone, GnRH has multiple functions including modulating neural activity within specific regions of the brain. These various functions are mediated by multiple isoforms, which are expressed at diverse locations within the central nervous system. Here we discuss the GnRH signaling pathways in light of new reports that reveal that some vertebrate genomes lack GnRH1. Not only do other isoforms of GnRH not compensate for this gene loss, but elements upstream of GnRH1, including kisspeptins, appear to also be dispensable. We discuss routes that may compensate for the loss of the GnRH1 pathway.
Keywords: Domestication; Evolution; Gene loss; Genome; GnIH; Kisspeptin; Synteny; Zebrafish.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Gore AC, GnRH: Tha Master Molecule of Reproduction, Kluwer Academic Publishers, Massachussetts, 2002.
-
- Lethimonier C, Madigou T, Munoz-Cueto JA, Lareyre JJ, and Kah O, Evolutionary aspects of GnRHs, GnRH neuronal systems and GnRH receptors in teleost fish. Gen Comp Endocrinol. 135 (2004) 1–16. - PubMed
-
- Kitahashi T, Shahjahan MD, and Parhar I, Hypothalamic Regulation of Pituitary Gonadotropins, Nova Science Publishers, Inc., 2013.
-
- Peter RE, Yu K-L, Marchant TA, and Rosenblum PM, Direct Regulation of the Teleost Adenohypophysis. Journal of Experimental Zoology Supplement (1990) 84–89.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

