Hindbrain astrocytes and glucose counter-regulation
- PMID: 30797812
- PMCID: PMC7145321
- DOI: 10.1016/j.physbeh.2019.02.025
Hindbrain astrocytes and glucose counter-regulation
Abstract
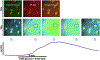
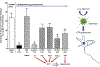
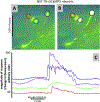
Hindbrain astrocytes are emerging as critical components in the regulation of homeostatic functions by either modulating synaptic activity or serving as primary detectors of physiological parameters. Recent studies have suggested that the glucose counter-regulation response (CRR), a critical defense against hypoglycemic emergencies, is dependent on glucoprivation-sensitive astrocytes in the hindbrain. This subpopulation of astrocytes produces a robust calcium signal in response to glucopenic stimuli. Both ex vivo and in vivo evidence suggest that low-glucose sensitive astrocytes utilize purinergic gliotransmission to activate catecholamine neurons in the hindbrain that are critical to the generation of the integrated CRR. Lastly, reports in the clinical literature suggest that an uncontrolled activation of CRR may as part of the pathology of severe traumatic injury. Work in our laboratory also suggests that this pathological hyperglycemia resulting from traumatic injury may be caused by the action of thrombin (generated by tissue trauma or bleeding) on hindbrain astrocytes. Similar to their glucopenia-sensitive neighbors, these hindbrain astrocytes may trigger hyperglycemic responses by their interactions with catecholaminergic neurons.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures



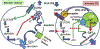
Similar articles
-
Evidence that hindbrain astrocytes in the rat detect low glucose with a glucose transporter 2-phospholipase C-calcium release mechanism.Am J Physiol Regul Integr Comp Physiol. 2020 Jan 1;318(1):R38-R48. doi: 10.1152/ajpregu.00133.2019. Epub 2019 Oct 9. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 31596114 Free PMC article.
-
Thrombin action on astrocytes in the hindbrain of the rat disrupts glycemic and respiratory control.Am J Physiol Regul Integr Comp Physiol. 2020 Jun 1;318(6):R1068-R1077. doi: 10.1152/ajpregu.00033.2020. Epub 2020 Apr 22. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 32320636 Free PMC article.
-
Response of catecholaminergic neurons in the mouse hindbrain to glucoprivic stimuli is astrocyte dependent.Am J Physiol Regul Integr Comp Physiol. 2018 Jul 1;315(1):R153-R164. doi: 10.1152/ajpregu.00368.2017. Epub 2018 Mar 28. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 29590557 Free PMC article.
-
Astrocytes in the hindbrain detect glucoprivation and regulate gastric motility.Auton Neurosci. 2013 Apr;175(1-2):61-9. doi: 10.1016/j.autneu.2012.12.006. Epub 2013 Jan 10. Auton Neurosci. 2013. PMID: 23313342 Free PMC article. Review.
-
Hindbrain catecholamine neurons control multiple glucoregulatory responses.Physiol Behav. 2006 Nov 30;89(4):490-500. doi: 10.1016/j.physbeh.2006.05.036. Epub 2006 Aug 1. Physiol Behav. 2006. PMID: 16887153 Review.
Cited by
-
Evidence that hindbrain astrocytes in the rat detect low glucose with a glucose transporter 2-phospholipase C-calcium release mechanism.Am J Physiol Regul Integr Comp Physiol. 2020 Jan 1;318(1):R38-R48. doi: 10.1152/ajpregu.00133.2019. Epub 2019 Oct 9. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 31596114 Free PMC article.
-
Hindbrain glucoregulatory mechanisms: Critical role of catecholamine neurons in the ventrolateral medulla.Physiol Behav. 2019 Sep 1;208:112568. doi: 10.1016/j.physbeh.2019.112568. Epub 2019 Jun 5. Physiol Behav. 2019. PMID: 31173784 Free PMC article. Review.
-
Shaking Table Test of a Transfer-Purge Chamber in Nuclear Island Structure.Materials (Basel). 2022 Jan 20;15(3):766. doi: 10.3390/ma15030766. Materials (Basel). 2022. PMID: 35160712 Free PMC article.
-
Astrocytes in the nucleus of the solitary tract: Contributions to neural circuits controlling physiology.Physiol Behav. 2020 Sep 1;223:112982. doi: 10.1016/j.physbeh.2020.112982. Epub 2020 Jun 11. Physiol Behav. 2020. PMID: 32535136 Free PMC article. Review.
-
Glial Modulation of Energy Balance: The Dorsal Vagal Complex Is No Exception.Int J Mol Sci. 2022 Jan 16;23(2):960. doi: 10.3390/ijms23020960. Int J Mol Sci. 2022. PMID: 35055143 Free PMC article. Review.
References
-
- Accorsi-Mendonca D, Bonagamba LGH, Machado BH, ATP released by glia increases the excitatory neurotransmission onto NTS neurons related to the peripheral chemoreflex, Soc. Neurosci 824 (809) (2012).
-
- Agulhon C, Fiacco TA, McCarthy KD, Hippocampal short- and long-term plas- ticity are not modulated by astrocyte Ca2+ signaling, Science 327 (2010) 1250–1254. - PubMed
-
- Andrew SF, Dinh TT, Ritter S, Localized glucoprivation of hindbrain sites elicits corticosterone and glucagon secretion, Am. J. Phys. Regul. Integr. Comp. Phys. 292 (2007) R1792–1798. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

