Effects of the antibiotic component on in-vitro bacterial killing, physico-chemical properties, aerosolization and dissolution of a ternary-combinational inhalation powder formulation of antibiotics for pan-drug resistant Gram-negative lung infections
- PMID: 30797863
- PMCID: PMC6441383
- DOI: 10.1016/j.ijpharm.2019.02.018
Effects of the antibiotic component on in-vitro bacterial killing, physico-chemical properties, aerosolization and dissolution of a ternary-combinational inhalation powder formulation of antibiotics for pan-drug resistant Gram-negative lung infections
Abstract
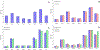
Combinational antibiotic formulations have emerged as an important strategy to combat antibiotic resistance. The main objective of this study was to examine effects of individual components on the antimicrobial activity, physico-chemical properties, aerosolization and dissolution of powder aerosol formulations when three synergistic drugs were co-spray dried. A ternary dry powder formulation consisting of meropenem (75.5 %w/w), colistin (15.1 %w/w) and rifampicin (9.4 %w/w) at the selected ratio was produced by spray drying. The ternary formulation was characterized for in-vitro antibacterial activity, physico-chemical properties, surface composition, aerosol performance and dissolution. All of the formulations demonstrated excellent aerosolization behavior achieving a fine particle fraction of >70%, which was substantially higher than those for the Meropenem-SD and Colistin-Meropenem formulations. The results indicated that rifampicin controlled the surface morphology of the ternary and binary combination formulations resulting in the formation of highly corrugated particles. Advanced characterization of surface composition by XPS supported the hypothesis that rifampicin was enriched on the surface of the combination powder formulations. All spray-dried formulations were amorphous and absorbed substantial amount of water at the elevated humidity. Storage at the elevated humidity caused a substantial decline in aerosolization performance for the Meropenem-SD and Colistin-Meropenem, which was attributed to increased inter-particulate capillary forces or particle fusion. In contrast, the ternary combination and binary Meropenem-Rifampicin formulations showed no change in aerosol performance at the elevated storage humidity conditions; attributable to the enriched hydrophobicity of rifampicin on the particle surface that acted as a barrier against moisture condensation and particle fusion. Interestingly, in the ternary formulation rifampicin enrichment on the surface did not interfere with the dissolution of other two components (i.e. meropenem and colistin). Our study provides an insight on the impact of each component on the performance of co-spray dried combinational formulations.
Keywords: Aerosol performance; Dissolution; Dry powder inhaler; Solubility; Spray drying; Ternary combination.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures




References
-
- Ambrose PG, Bhavnani SM, Grosse EJE, Drusano GL, 2010. Pharmacokinetic-Pharmacodynamic Considerations in the Design of Hospital-Acquired or Ventilator-Associated Bacterial Pneumonia Studies: Look before You Leap! Clinical Infectious Diseases 51, S103–S110. - PubMed
-
- Bohr A, P Boetker J, Rades T, Rantanen J, Yang M, 2014. Application of spray-drying and electrospraying/electospinning for poorly watersoluble drugs: A particle engineering approach. Current pharmaceutical design 20, 325–348. - PubMed
-
- Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J, 2009. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clinical Infectious Diseases 48, 1–12. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

