Chronopharmacology of glucocorticoids
- PMID: 30797955
- PMCID: PMC6703983
- DOI: 10.1016/j.addr.2019.02.004
Chronopharmacology of glucocorticoids
Abstract
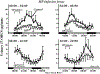
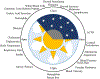
Glucocorticoids influence a wide array of metabolic, anti-inflammatory, immunosuppressive, and cognitive signaling processes, playing an important role in homeostasis and preservation of normal organ function. Synthesis is regulated by the hypothalamic-pituitary-adrenal (HPA) axis of which cortisol is the primary glucocorticoid in humans. Synthetic glucocorticoids are important pharmacological agents that augment the anti-inflammatory and immunosuppressive properties of endogenous cortisol and are widely used for the treatment of asthma, Crohn's disease, and rheumatoid arthritis, amongst other chronic conditions. The homeostatic activity of cortisol is disrupted by the administration of synthetic glucocorticoids and so there is interest in developing treatment options that minimize HPA axis disturbance while maintaining the pharmacological effects. Studies suggest that optimizing drug administration time can achieve this goal. The present review provides an overview of endogenous glucocorticoid activity and recent advances in treatment options that have further improved patient safety and efficacy with an emphasis on chronopharmacology.
Keywords: Chronopharmacokinetics; Chronopharmacology; Circadian rhythms; Cortisol; HPA axis disruption; Synthetic glucocorticoids.
Copyright © 2019. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Interest Statement
The authors have no conflicts of interest to declare.
Figures






References
-
- Fietta P, Delsante G, Central nervous system effects of natural and synthetic glucocorticoids, Psychiatry Clin Neurosci, 63 (2009) 613–622. - PubMed
-
- Arlt W, Stewart PM, Adrenal corticosteroid biosynthesis, metabolism, and action, Endocrinol Metab Clin North Am, 34 (2005) 293–313, viii. - PubMed
-
- Meduri GU, Annane D, Chrousos GP, Marik PE, Sinclair SE, Activation and regulation of systemic inflammation in ARDS: rationale for prolonged glucocorticoid therapy, Chest, 136 (2009) 1631–1643. - PubMed
-
- Elenkov IJ, Chrousos GP, Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity, Ann N Y Acad Sci, 966 (2002) 290–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

