α-Synuclein and astrocytes: tracing the pathways from homeostasis to neurodegeneration in Lewy body disease
- PMID: 30798354
- PMCID: PMC6571045
- DOI: 10.1007/s00401-019-01977-2
α-Synuclein and astrocytes: tracing the pathways from homeostasis to neurodegeneration in Lewy body disease
Abstract
α-Synuclein is a soluble protein that is present in abundance in the brain, though its normal function in the healthy brain is poorly defined. Intraneuronal inclusions of α-synuclein, commonly referred to as Lewy pathology, are pathological hallmarks of a spectrum of neurodegenerative disorders referred to as α-synucleinopathies. Though α-synuclein is expressed predominantly in neurons, α-synuclein aggregates in astrocytes are a common feature in these neurodegenerative diseases. How and why α-synuclein ends up in the astrocytes and the consequences of this dysfunctional proteostasis in immune cells is a major area of research that can have far-reaching implications for future immunobiotherapies in α-synucleinopathies. Accumulation of aggregated α-synuclein can disrupt astrocyte function in general and, more importantly, can contribute to neurodegeneration in α-synucleinopathies through various pathways. Here, we summarize our current knowledge on how astrocytic α-synucleinopathy affects CNS function in health and disease and propose a model of neuroglial connectome altered by α-synuclein proteostasis that might be amenable to immune-based therapies.
Keywords: Astrocyte heterogeneity; Exosome; Glial cytoplasmic inclusion; Lewy body; NAC domain; Neurodegeneration; Therapy; Transmission; Tunneling nanotube; α-Synuclein; αSyn 3H11.
Figures

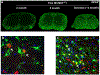
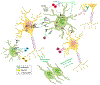
References
-
- Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ (2006) Phosphorylation of Ser-129 Is the Dominant Pathological Modification of α-Synuclein in Familial and Sporadic Lewy Body Disease. J Biol Chem 281:29739–29752. doi: 10.1074/jbc.M600933200 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

