Recruitment of RNA Polymerase II to Metabolic Gene Promoters Is Inhibited in the Failing Heart Possibly Through PGC-1α (Peroxisome Proliferator-Activated Receptor-γ Coactivator-1α) Dysregulation
- PMID: 30798619
- PMCID: PMC6392084
- DOI: 10.1161/CIRCHEARTFAILURE.118.005529
Recruitment of RNA Polymerase II to Metabolic Gene Promoters Is Inhibited in the Failing Heart Possibly Through PGC-1α (Peroxisome Proliferator-Activated Receptor-γ Coactivator-1α) Dysregulation
Abstract
Background: Proper dynamics of RNA polymerase II, such as promoter recruitment and elongation, are essential for transcription. PGC-1α (peroxisome proliferator-activated receptor [PPAR]-γ coactivator-1α), also termed PPARGC1a, is a transcriptional coactivator that stimulates energy metabolism, and PGC-1α target genes are downregulated in the failing heart. However, whether the dysregulation of polymerase II dynamics occurs in PGC-1α target genes in heart failure has not been defined.
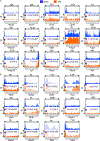
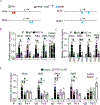
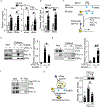
Methods and results: Chromatin immunoprecipitation-sequencing revealed that reduced promoter occupancy was a major form of polymerase II dysregulation on PGC-1α target metabolic gene promoters in the pressure-overload-induced heart failure model. PGC-1α-cKO (cardiac-specific PGC-1α knockout) mice showed phenotypic similarity to the pressure-overload-induced heart failure model in wild-type mice, such as contractile dysfunction and downregulation of PGC-1α target genes, even under basal conditions. However, the protein levels of PGC-1α were neither changed in the pressure-overload model nor in human failing hearts. Chromatin immunoprecipitation assays revealed that the promoter occupancy of polymerase II and PGC-1α was consistently reduced both in the pressure-overload model and PGC-1α-cKO mice. In vitro DNA binding assays using an endogenous PGC-1α target gene promoter sequence confirmed that PGC-1α recruits polymerase II to the promoter.
Conclusions: These results suggest that PGC-1α promotes the recruitment of polymerase II to the PGC-1α target gene promoters. Downregulation of PGC-1α target genes in the failing heart is attributed, in part, to a reduction of the PGC-1α occupancy and the polymerase II recruitment to the promoters, which might be a novel mechanism of metabolic perturbations in the failing heart.
Keywords: RNA polymerase II; chromatin; energy metabolism; heart failure.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

