hESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids
- PMID: 30799279
- PMCID: PMC6853597
- DOI: 10.1016/j.stem.2018.12.015
hESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids
Abstract
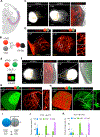
Human brain organoid techniques have rapidly advanced to facilitate investigating human brain development and diseases. These efforts have largely focused on generating telencephalon due to its direct relevance in a variety of forebrain disorders. Despite its importance as a relay hub between cortex and peripheral tissues, the investigation of three-dimensional (3D) organoid models for the human thalamus has not been explored. Here, we describe a method to differentiate human embryonic stem cells (hESCs) to thalamic organoids (hThOs) that specifically recapitulate the development of thalamus. Single-cell RNA sequencing revealed a formation of distinct thalamic lineages, which diverge from telencephalic fate. Importantly, we developed a 3D system to create the reciprocal projections between thalamus and cortex by fusing the two distinct region-specific organoids representing the developing thalamus or cortex. Our study provides a platform for understanding human thalamic development and modeling circuit organizations and related disorders in the brain.
Keywords: brain organoid; cortex; corticothalamic; fusion; hESC; single cell RNA-seq; thalamic organoid; thalamocortical; thalamus.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

