Poly(ADP-ribosyl)ation by PARP1: reaction mechanism and regulatory proteins
- PMID: 30799503
- PMCID: PMC6486540
- DOI: 10.1093/nar/gkz120
Poly(ADP-ribosyl)ation by PARP1: reaction mechanism and regulatory proteins
Abstract
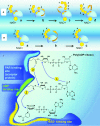
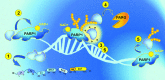
Poly(ADP-ribosyl)ation (PARylation) is posttranslational modification of proteins by linear or branched chains of ADP-ribose units, originating from NAD+. The central enzyme for PAR production in cells and the main target of poly(ADP-ribosyl)ation during DNA damage is poly(ADP-ribose) polymerase 1 (PARP1). PARP1 ability to function as a catalytic and acceptor protein simultaneously made a considerable contribution to accumulation of contradictory data. This topic is directly related to other questions, such as the stoichiometry of PARP1 molecules in auto-modification reaction, direction of the chain growth during PAR elongation and functional coupling of PARP1 with PARylation targets. Besides DNA damage necessary for the folding of catalytically active PARP1, other mechanisms appear to be required for the relevant intensity and specificity of PARylation reaction. Indeed, in recent years, PARP research has been enriched by the discovery of novel PARP1 interaction partners modulating its enzymatic activity. Understanding the details of PARP1 catalytic mechanism and its regulation is especially important in light of PARP-targeted therapy and may significantly aid to PARP inhibitors drug design. In this review we summarize old and up-to-date literature to clarify several points concerning PARylation mechanism and discuss different ways for regulation of PAR synthesis by accessory proteins reported thus far.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Hassa P.O., Hottiger M.O.. The diverse biological roles of mammalian PARPS, a small but powerful family of poly-ADP-ribose polymerases. Front. Biosci. 2008; 13:3046–3082. - PubMed
-
- Kleine H., Poreba E., Lesniewicz K., Hassa P.O., Hottiger M.O., Litchfield D.W., Shilton B.H., Lüscher B.. Substrate-assisted catalysis by PARP10 limits its activity to mono-ADP-ribosylation. Mol. Cell. 2008; 32:57–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

