The Role of Maternal Thyroid Hormones in Avian Embryonic Development
- PMID: 30800099
- PMCID: PMC6375826
- DOI: 10.3389/fendo.2019.00066
The Role of Maternal Thyroid Hormones in Avian Embryonic Development
Abstract
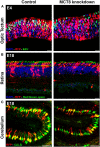
During avian embryonic development, thyroid hormones (THs) coordinate the expression of a multitude of genes thereby ensuring that the correct sequence of cell proliferation, differentiation and maturation is followed in each tissue and organ. Although THs are needed from the start of development, the embryonic thyroid gland only matures around mid-incubation in precocial birds and around hatching in altricial species. Therefore, maternal THs deposited in the egg yolk play an essential role in embryonic development. They are taken up by the embryo throughout its development, from the first day till hatching, and expression of TH regulators such as distributor proteins, transporters, and deiodinases in the yolk sac membrane provide the tools for selective metabolism and transport starting from this level. TH receptors and regulators of local TH availability are expressed in avian embryos in a dynamic and tissue/cell-specific pattern from the first stages studied, as shown in detail in chicken. Maternal hyperthyroidism via TH supplementation as well as injection of THs into the egg yolk increase TH content in embryonic tissues while induction of maternal hypothyroidism by goitrogen treatment results in a decrease. Both increase and decrease of maternal TH availability were shown to alter gene expression in early chicken embryos. Knockdown of the specific TH transporter monocarboxylate transporter 8 at early stages in chicken cerebellum, optic tectum, or retina allowed to reduce local TH availability, interfering with gene expression and confirming that development of the central nervous system (CNS) is highly dependent on maternal THs. While some of the effects on cell proliferation, migration and differentiation seem to be transient, others result in persistent defects in CNS structure. In addition, a number of studies in both precocial and altricial birds showed that injection of THs into the yolk at the start of incubation influences a number of parameters in posthatch performance and fitness. In conclusion, the data presently available clearly indicate that maternal THs play an important role in avian embryonic development, but how exactly their influence on cellular and molecular processes in the embryo is linked to posthatch fitness needs to be further explored.
Keywords: TH transporter; bird; deiodinase; development; thyroid hormone.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

