Synthesis of 1,2-divinylcyclopropanes by metal-catalyzed cyclopropanation of 1,3-dienes with cyclopropenes as vinyl carbene precursors
- PMID: 30800178
- PMCID: PMC6369990
- DOI: 10.3762/bjoc.15.25
Synthesis of 1,2-divinylcyclopropanes by metal-catalyzed cyclopropanation of 1,3-dienes with cyclopropenes as vinyl carbene precursors
Abstract
The synthesis of 1,2-divinylcyclopropanes by the reaction of cyclopropenes with 1,3-dienes is reported. The process relies on the ability of ZnCl2 or [Rh2(OAc)4] to generate metal-vinyl carbene intermediates from cyclopropenes, which effect cyclopropanation of 1,3-dienes. Most of the reactions proceeded in reasonable yields while the diastereoselectivity strongly depends on the structure of the diene. An example of an intramolecular process as well as the use of furan and 1,4-cyclohexadiene as dienes are also reported.
Keywords: cyclopropanation; cyclopropenes; dienes; divinylcyclopropanes; transition metal catalysis.
Figures


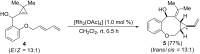

Similar articles
-
Intramolecular cyclopropanation and C-H insertion reactions with metal carbenoids generated from cyclopropenes.Acc Chem Res. 2015 Apr 21;48(4):1021-31. doi: 10.1021/acs.accounts.5b00016. Epub 2015 Mar 12. Acc Chem Res. 2015. PMID: 25763601
-
A Dinickel Catalyzed Cyclopropanation without the Formation of a Metal Carbene Intermediate.Angew Chem Int Ed Engl. 2021 Jan 25;60(4):1897-1902. doi: 10.1002/anie.202011602. Epub 2020 Nov 23. Angew Chem Int Ed Engl. 2021. PMID: 33045127 Free PMC article.
-
Catalytic cyclopropanation of alkenes via (2-furyl)carbene complexes from 1-benzoyl-cis-1-buten-3-yne with transition metal compounds.J Org Chem. 2004 Mar 5;69(5):1557-64. doi: 10.1021/jo0352732. J Org Chem. 2004. PMID: 14987011
-
Synthesis of Functionalized α-Vinyl Aldehydes from Enaminones.Angew Chem Int Ed Engl. 2019 Sep 2;58(36):12674-12679. doi: 10.1002/anie.201906213. Epub 2019 Aug 1. Angew Chem Int Ed Engl. 2019. PMID: 31278817 Review.
-
Dienes as Versatile Substrates for Transition Metal-Catalyzed Reactions.Angew Chem Int Ed Engl. 2024 May 13;63(20):e202401550. doi: 10.1002/anie.202401550. Epub 2024 Mar 19. Angew Chem Int Ed Engl. 2024. PMID: 38436553 Free PMC article. Review.
Cited by
-
Gold-Catalyzed Synthesis of Small Rings.Chem Rev. 2021 Jul 28;121(14):8613-8684. doi: 10.1021/acs.chemrev.0c00697. Epub 2020 Nov 2. Chem Rev. 2021. PMID: 33136374 Free PMC article.
-
Assembly of Complex 1,4-Cycloheptadienes by (4+3) Cycloaddition of Rhodium(II) and Gold(I) Non-Acceptor Carbenes.Angew Chem Int Ed Engl. 2021 Jan 25;60(4):1916-1922. doi: 10.1002/anie.202012092. Epub 2020 Nov 24. Angew Chem Int Ed Engl. 2021. PMID: 33078893 Free PMC article.
References
-
- Donaldson W A. Tetrahedron. 2001;57:8589–8627. doi: 10.1016/s0040-4020(01)00777-3. - DOI
LinkOut - more resources
Full Text Sources
