Sigmatropic rearrangements of cyclopropenylcarbinol derivatives. Access to diversely substituted alkylidenecyclopropanes
- PMID: 30800182
- PMCID: PMC6369978
- DOI: 10.3762/bjoc.15.29
Sigmatropic rearrangements of cyclopropenylcarbinol derivatives. Access to diversely substituted alkylidenecyclopropanes
Abstract
Cyclopropenes constitute useful precursors of other classes of compounds incorporating a three-membered ring. Although the transformation of substituted cyclopropenes into alkylidenecyclopropanes can be accomplished through different strategies, this review is focusing specifically on the use of [2,3]- and [3,3]-sigmatropic rearrangements involving cyclopropenylcarbinol derivatives as substrates. These sigmatropic rearrangements, which have been developed in recent years, allow a remarkably efficient and stereoselective access to a wide variety of heterosubstituted and/or functionalized alkylidenecyclopropanes which would not be readily accessible by other strategies. The different [2,3]- and [3,3]-sigmatropic rearrangements of cyclopropenylcarbinol derivatives disclosed to date, as well as the analysis of their substrate scope and some applications of the products arising from those reactions, are presented in this review.
Keywords: alkylidenecyclopropanes; cyclopropanes; cyclopropenes; sigmatropic rearrangements; strained rings.
Figures


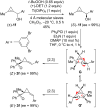
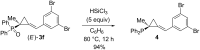

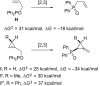

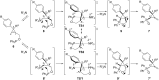


















References
-
- Vicente R. Synthesis. 2016;48:2343–2360. doi: 10.1055/s-0035-1561644. - DOI
Publication types
LinkOut - more resources
Full Text Sources
