Tandem copper and photoredox catalysis in photocatalytic alkene difunctionalization reactions
- PMID: 30800183
- PMCID: PMC6369989
- DOI: 10.3762/bjoc.15.30
Tandem copper and photoredox catalysis in photocatalytic alkene difunctionalization reactions
Abstract
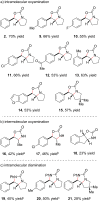
Oxidative alkene difunctionalization reactions are important in synthetic organic chemistry because they can install polar functional groups onto simple non-polar alkene moieties. Many of the most common methods for these reactions rely upon the reactivity of pre-oxidized electrophilic heteroatom donors that can often be unstable, explosive, or difficult to handle. Herein, we describe a method for alkene oxyamination and diamination that utilizes simple carbamate and urea groups as nucleophilic heteroatom donors. This method uses a tandem copper-photoredox catalyst system that is operationally convenient. The identity of the terminal oxidant is critical in these studies. Ag(I) salts proved to be unique in their ability to turn over the copper cocatalyst without deleteriously impacting the reactivity of the organoradical intermediates.
Keywords: copper; diamination; oxidative functionalization; oxyamination; photoredox catalysis; radical.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
