Safety and effectiveness of FOLFOXIRI plus molecular target drug therapy for metastatic colorectal cancer: A multicenter retrospective study
- PMID: 30800219
- PMCID: PMC6383688
- DOI: 10.18632/oncotarget.26626
Safety and effectiveness of FOLFOXIRI plus molecular target drug therapy for metastatic colorectal cancer: A multicenter retrospective study
Abstract
Introduction: FOLFOXIRI plus bevacizumab has a promising efficacy as first-line systemic chemotherapy for metastatic colorectal cancer (mCRC). This study aimed to evaluate the safety and effectiveness of FOLFOXIRI plus antibodies.
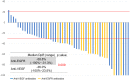
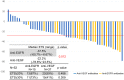
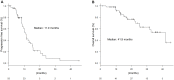
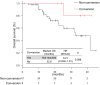
Results: Fifty-five patients were enrolled (median age: 60 years, males: 25, females: 30). Twenty-six subjects had RAS mutations and 29 had RAS wild-type. Anti-VEGF and anti-EGFR antibodies were administered to 38 and 17 patients, respectively. The most common severe adverse event was neutropenia (51%). The overall response rate (ORR) was 69% (55% with anti-VEGF antibodies and 100% with anti-EGFR antibodies; P = 0.190), and the disease control rate was 98% (stable disease: 16 patients). With a median follow-up period of 18.4 months, the median progression-free survival (mPFS) was 11.0 months and the median overall survival (mOS) was 41.9 months. The mPFS and mOS did not significantly differ between patients treated with anti-EGFR antibodies and those with anti-VEGF antibodies.
Methods: We retrospectively collected data from mCRC patients treated with FOLFOXIRI plus antibodies between March 2014 and December 2017.
Conclusions: FOLFOXIRI plus antibody therapy was effective in patients with mCRC. The response rate was higher in patients treated with anti-EGFR antibodies than in those treated with anti-VEGF antibodies.
Keywords: FOLFOXIRI therapy; anti-EGFR antibodies; anti-VEGF antibodies; metastatic colorectal cancer; triplet.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures


Similar articles
-
FOLFOXIRI-Bevacizumab or FOLFOX-Panitumumab in Patients with Left-Sided RAS/BRAF Wild-Type Metastatic Colorectal Cancer: A Propensity Score-Based Analysis.Oncologist. 2021 Apr;26(4):302-309. doi: 10.1002/onco.13642. Epub 2021 Jan 2. Oncologist. 2021. PMID: 33336844 Free PMC article.
-
Protocol of the EFFORT study: a prospective study of FOLFIRI plus aflibercept as second-line treatment after progression on FOLFOXIRI plus bevacizumab or during maintenance treatment in patients with unresectable/metastatic colorectal cancer.BMC Cancer. 2020 Nov 17;20(1):1116. doi: 10.1186/s12885-020-07576-9. BMC Cancer. 2020. PMID: 33203393 Free PMC article.
-
Phase II Randomized Trial of Sequential or Concurrent FOLFOXIRI-Bevacizumab Versus FOLFOX-Bevacizumab for Metastatic Colorectal Cancer (STEAM).Oncologist. 2019 Jul;24(7):921-932. doi: 10.1634/theoncologist.2018-0344. Epub 2018 Dec 14. Oncologist. 2019. PMID: 30552157 Free PMC article. Clinical Trial.
-
Monoclonal antibodies in the treatment of metastatic colorectal cancer: a review.Clin Ther. 2010 Mar;32(3):437-53. doi: 10.1016/j.clinthera.2010.03.012. Clin Ther. 2010. PMID: 20399983 Review.
-
Triplet chemotherapy in combination with anti-EGFR agents for the treatment of metastatic colorectal cancer: Current evidence, advances, and future perspectives.Cancer Treat Rev. 2022 Jan;102:102301. doi: 10.1016/j.ctrv.2021.102301. Epub 2021 Nov 11. Cancer Treat Rev. 2022. PMID: 34839118 Review.
Cited by
-
Ursolic acid induces apoptosis and anoikis in colorectal carcinoma RKO cells.BMC Complement Med Ther. 2021 Feb 6;21(1):52. doi: 10.1186/s12906-021-03232-2. BMC Complement Med Ther. 2021. PMID: 33549076 Free PMC article.
-
Is there an efficacy-effectiveness gap between randomized controlled trials and real-world studies in colorectal cancer: a systematic review and meta-analysis.Transl Cancer Res. 2020 Nov;9(11):6963-6987. doi: 10.21037/tcr-20-2303. Transl Cancer Res. 2020. PMID: 35117304 Free PMC article.
-
Efficacy and safety of triplet chemotherapy plus anti-EGFR agents in metastatic colorectal cancer: a systematic review and meta-analysis.World J Surg Oncol. 2022 Aug 15;20(1):258. doi: 10.1186/s12957-022-02707-x. World J Surg Oncol. 2022. PMID: 35965307 Free PMC article.
References
-
- Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmuller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–75. doi: 10.1016/S1470-2045(14)70330-4. - DOI - PubMed
-
- Yamada Y, Denda T, Gamoh M, Iwanaga I, Yuki S, Shimodaira H, Nakamura M, Yamaguchi T, Ohori H, Kobayashi K, Tsuda M, Kobayashi Y, Miyamoto Y, et al. S-1 and irinotecan plus bevacizumab versus mFOLFOX6 or CapeOX plus bevacizumab as first-line treatment in patients with metastatic colorectal cancer (TRICOLORE): a randomized, open-label, phase III, noninferiority trial. Ann Oncol. 2018;29:624–31. doi: 10.1093/annonc/mdx816. - DOI - PMC - PubMed
-
- Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R, Zaniboni A, Tonini G, Buonadonna A, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371:1609–18. doi: 10.1056/NEJMoa1403108. - DOI - PubMed
-
- Satake H, Sunakawa Y, Miyamoto Y, Nakamura M, Nakayama H, Shiozawa M, Makiyama A, Kobayashi K, Kubota Y, Mori M, Kotaka M, Takagane A, Gotoh M, et al. A phase II trial of 1st-line modified-FOLFOXIRI plus bevacizumab treatment for metastatic colorectal cancer harboring RAS mutation: JACCRO CC-11. Oncotarget. 2018;9:18811–20. doi: 10.18632/oncotarget.24702. - DOI - PMC - PubMed
-
- Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, Heinemann V, Van Cutsem E, Pignon JP, Tabernero J, Cervantes A, Ciardiello F. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713–29. doi: 10.1093/annonc/mdx175. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

