Repurposing old carbon monoxide-releasing molecules towards the anti-angiogenic therapy of triple-negative breast cancer
- PMID: 30800223
- PMCID: PMC6383690
- DOI: 10.18632/oncotarget.26638
Repurposing old carbon monoxide-releasing molecules towards the anti-angiogenic therapy of triple-negative breast cancer
Abstract
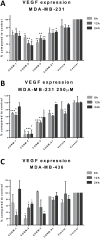
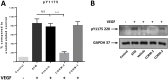
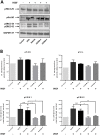
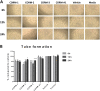
Triple-negative breast cancer (TNBC) is defined by the lack of expression of the oestrogen and progesterone receptors and HER-2. Recently, carbon monoxide (CO) was found to behave as an important endogenous signalling molecule and to suppress VEGF receptor-2 (VEGFR-2) and protein kinase B phosphorylation. Given that anti-angiogenic drugs exist as one of the few available targeted therapies against TNBC, the aim of this project was to study the effects of CO-releasing molecules (CORMs) on TNBC cell lines and the associated endothelial cells and characterise their anti-angiogenic properties that can be used for the reduction of cancer-driven angiogenesis. Four commercially available CORMs were screened for their cytotoxicity, their effects on cell metabolism, migration, VEGF expression, tube formation and VEGFR-2 activation. The most important result was the reduction in VEGF levels expressed by CORM-treated TNBC cells, along with the inhibition of phosphorylation of VEGFR2 and downstream proteins. The migration and tube formation ability of endothelial cells was also decreased by CORMs, justifying a potential re-purposing of old CORMs towards the anti-angiogenic therapy of TNBC. The additional favourable low cytotoxicity, reduction in the glycolysis levels and downregulation of haem oxygenase-1 in TNBC cells enhance the potential of CORMs against TNBC. In this study, CORM-2 remained the most effective CORM and we propose that CORM-2 may be pursued further as an additional agent in combination with existing anti-angiogenic therapies for a more successful targeting of malignant angiogenesis in TNBC.
Keywords: angiogenesis; anti-angiogenic therapy; breast cancer; carbon-monoxide releasing molecules (CORMs); triple-negative breast cancer (TNBC).
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no potential conflicts of interest.
Figures


Similar articles
-
Structural Modifications on CORM-3 Lead to Enhanced Anti-angiogenic Properties Against Triple-negative Breast Cancer Cells.Med Chem. 2021;17(1):40-59. doi: 10.2174/1573406415666191206102452. Med Chem. 2021. PMID: 31808392
-
Co-targeting triple-negative breast cancer cells and endothelial cells by metronomic chemotherapy inhibits cell regrowth and migration via downregulation of the FAK/VEGFR2/VEGF axis and autophagy/apoptosis activation.Front Oncol. 2022 Nov 30;12:998274. doi: 10.3389/fonc.2022.998274. eCollection 2022. Front Oncol. 2022. PMID: 36531071 Free PMC article.
-
Vascular and angiogenic activities of CORM-401, an oxidant-sensitive CO-releasing molecule.Biochem Pharmacol. 2016 Feb 15;102:64-77. doi: 10.1016/j.bcp.2015.12.014. Epub 2015 Dec 22. Biochem Pharmacol. 2016. PMID: 26721585
-
Carbon Monoxide and Its Controlled Release: Therapeutic Application, Detection, and Development of Carbon Monoxide Releasing Molecules (CORMs).J Med Chem. 2018 Apr 12;61(7):2611-2635. doi: 10.1021/acs.jmedchem.6b01153. Epub 2017 Sep 20. J Med Chem. 2018. PMID: 28876065 Review.
-
Water-Soluble Carbon Monoxide-Releasing Molecules (CORMs).Top Curr Chem (Cham). 2022 Dec 14;381(1):3. doi: 10.1007/s41061-022-00413-6. Top Curr Chem (Cham). 2022. PMID: 36515756 Review.
Cited by
-
Carbon monoxide: An emerging therapy for acute kidney injury.Med Res Rev. 2020 Jul;40(4):1147-1177. doi: 10.1002/med.21650. Epub 2019 Dec 9. Med Res Rev. 2020. PMID: 31820474 Free PMC article. Review.
-
Gasotransmitters in the tumor microenvironment: Impacts on cancer chemotherapy (Review).Mol Med Rep. 2022 Jul;26(1):233. doi: 10.3892/mmr.2022.12749. Epub 2022 May 26. Mol Med Rep. 2022. PMID: 35616143 Free PMC article. Review.
-
DNA damage and antioxidant properties of CORM-2 in normal and cancer cells.Sci Rep. 2020 Jul 22;10(1):12200. doi: 10.1038/s41598-020-68948-6. Sci Rep. 2020. PMID: 32699258 Free PMC article.
-
Trends and Challenges in Tumor Anti-Angiogenic Therapies.Cells. 2019 Sep 18;8(9):1102. doi: 10.3390/cells8091102. Cells. 2019. PMID: 31540455 Free PMC article. Review.
-
Carbon monoxide-triggered health effects: the important role of the inflammasome and its possible crosstalk with autophagy and exosomes.Arch Toxicol. 2021 Apr;95(4):1141-1159. doi: 10.1007/s00204-021-02976-7. Epub 2021 Feb 8. Arch Toxicol. 2021. PMID: 33554280 Review.
References
-
- Wehland M, Bauer J, Infanger M, Grimm D. Target-based anti-angiogenic therapy in breast cancer. Curr Pharm Des. 2012;18:4244–4257. - PubMed
-
- Kalimutho M, Parsons K, Mittal D, López JA, Srihari S, Khanna KK. Targeted Therapies for Triple-Negative Breast Cancer: Combating a Stubborn Disease. Trends Pharmacol Sci. 2015;36:822–846. - PubMed
-
- Fosu-Mensah N, Peris MS, Weeks HP, Cai J, Westwell AD. Advances in small-molecule drug discovery for triple-negative breast cancer. Future Med Chem. 2015;7:2019–2039. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

