Structural modification of isomorphous SO4 2--doped K2FeO4 for remediating the stability and enhancing the discharge of super-iron battery
- PMID: 30800350
- PMCID: PMC6366229
- DOI: 10.1098/rsos.180919
Structural modification of isomorphous SO4 2--doped K2FeO4 for remediating the stability and enhancing the discharge of super-iron battery
Abstract
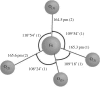
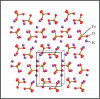
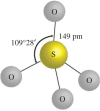
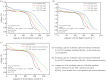
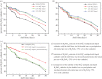
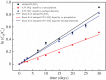
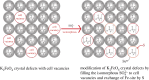
In the paper, the isomorphous doped K2FeO4, aimed at the remediation of the discharge and stability of the super-iron battery, was first synthesized for doping and reforming the K2FeO4 crystalline structure via a facile co-precipitation and mechanochemistry. Afterwards, the compared cathodes were assembled by the undoped and doped K2FeO4 for an evaluation of the discharge and stability in the AAA super-iron battery system. The results show that the small amounts of K2SO4 were doped into the K2FeO4 in the calculated form of K2Fe1-xSxO4 by the isomorphous substitution. The doped K2FeO4 cathodes/batteries exhibited an excellent discharge with a normal discharge profile. The cathodes doped by two techniques had significantly enhanced the discharge capacity of the super-iron battery with an increase of 10-30% compared to the undoped K2FeO4. Moreover, the stability of the K2FeO4 cathodes was obviously remediated by the isomorphous doping. The shelf time of the doped K2FeO4 cathodes was prolonged by an increase of about 10% in comparison of the undoped K2FeO4 cathode. The desirable enhancements could be attributed to doping and reforming the similar building block and isomorphous into the tetrahedral and crystalline in the form of the isomorphous substitution and filling vacancies.
Keywords: K2FeO4; capacity; discharge; ferrates; stability; super-iron battery.
Conflict of interest statement
We declare no competing interests.
Figures


Similar articles
-
Er-Doped LiNi0.5Mn1.5O₄ Cathode Material with Enhanced Cycling Stability for Lithium-Ion Batteries.Materials (Basel). 2017 Jul 27;10(8):859. doi: 10.3390/ma10080859. Materials (Basel). 2017. PMID: 28773224 Free PMC article.
-
Nitrogen-Doped Perovskite as a Bifunctional Cathode Catalyst for Rechargeable Lithium-Oxygen Batteries.ACS Appl Mater Interfaces. 2018 Feb 14;10(6):5543-5550. doi: 10.1021/acsami.7b17289. Epub 2018 Jan 30. ACS Appl Mater Interfaces. 2018. PMID: 29338167
-
Understanding the influence of Mg doping for the stabilization of capacity and higher discharge voltage of Li- and Mn-rich cathodes for Li-ion batteries.Phys Chem Chem Phys. 2017 Feb 22;19(8):6142-6152. doi: 10.1039/c6cp07383b. Phys Chem Chem Phys. 2017. PMID: 28191568
-
Enhanced electrochemical properties of LiFePO4 by Mo-substitution and graphitic carbon-coating via a facile and fast microwave-assisted solid-state reaction.Phys Chem Chem Phys. 2012 Mar 14;14(10):3634-9. doi: 10.1039/c2cp24062a. Epub 2012 Feb 7. Phys Chem Chem Phys. 2012. PMID: 22311165
-
Iron-Doped Sodium Vanadium Oxyflurophosphate Cathodes for Sodium-Ion Batteries-Electrochemical Characterization and In Situ Measurements of Heat Generation.ACS Appl Mater Interfaces. 2020 Sep 16;12(37):41765-41775. doi: 10.1021/acsami.0c11616. Epub 2020 Sep 3. ACS Appl Mater Interfaces. 2020. PMID: 32809791
References
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

