Crystal structure and Hirshfeld surface analysis of (E)-1-[2,2-di-chloro-1-(4-nitro-phen-yl)ethen-yl]-2-(4-fluoro-phen-yl)diazene
- PMID: 30800458
- PMCID: PMC6362658
- DOI: 10.1107/S2056989019000707
Crystal structure and Hirshfeld surface analysis of (E)-1-[2,2-di-chloro-1-(4-nitro-phen-yl)ethen-yl]-2-(4-fluoro-phen-yl)diazene
Abstract
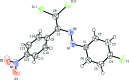
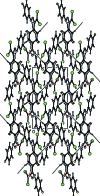
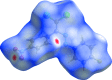
In the title compound, C14H8Cl2FN3O2, the 4-fluoro-phenyl ring and the nitro-substituted benzene ring form a dihedral angle of 63.29 (8)°. In the crystal, mol-ecules are linked by C-H⋯O hydrogen bonds into chains running parallel to the c axis. The crystal packing is further stabilized by C-Cl⋯π, C-F⋯π and N-O⋯π inter-actions. The Hirshfeld surface analysis of the crystal structure indicates that the most important contributions to the crystal packing are from H⋯O/O⋯H (15.5%), H⋯H (15.3%), Cl⋯H/H⋯Cl (13.8%), C⋯H/H⋯C (9.5%) and F⋯H/H⋯F (8.2%) inter-actions.
Keywords: 4-fluorophenyl ring; Hirshfeld surface analysis; crystal structure; hydrogen bonding; nitro-substituted benzene ring.
Figures

References
-
- Akbari Afkhami, F., Mahmoudi, G., Gurbanov, A. V., Zubkov, F. I., Qu, F., Gupta, A. & Safin, D. A. (2017). Dalton Trans. 46, 14888–14896. - PubMed
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
-
- Borisova, K. K., Nikitina, E. V., Novikov, R. A., Khrustalev, V. N., Dorovatovskii, P. V., Zubavichus, Y. V., Kuznetsov, M. L., Zaytsev, V. P., Varlamov, A. V. & Zubkov, F. I. (2018). Chem. Commun. 54, 2850–2853. - PubMed
-
- Bruker (2003). SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Bruker (2007). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
