Crystal structure of a host-guest complex between mephedrone hydro-chloride and a tetra-phospho-nate cavitand
- PMID: 30800467
- PMCID: PMC6362660
- DOI: 10.1107/S2056989019001464
Crystal structure of a host-guest complex between mephedrone hydro-chloride and a tetra-phospho-nate cavitand
Abstract
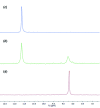
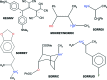
A new supra-molecular complex (I) between the tetra-phospho-nate cavitand Tiiii[C3H7,CH3,C6H5] [systematic name: 2,8,14,20-tetra-propyl-5,11,17,23-tetra-methyl-6,10:12,16:18,22:24,4-tetra-kis-(phenyl-phospho-nato-O,O')resorcin[4]arene] and mephedrone hydro-choride {C11H16NO+·Cl-; systematic name: meth-yl[1-(4-methyl-phen-yl)-1-oxopropan-2-yl]aza-nium chloride} has been obtained and characterized both in solution and in the solid state. The complex of general formula (C11H16NO)@Tiiii[C3H7,CH3,C6H5]Cl·CH3OH or C11H16NO+·Cl-·C68H68O12P4·CH3OH, crystallizes in the monoclinic space group P21/c with one lattice methanol mol-ecule per cavitand, disordered over two positions with occupancy factors of 0.665 (6) and 0.335 (6). The mephedrone guest inter-acts with the P=O groups at the upper rim of the cavitand through two charge-assisted N-H⋯O hydrogen bonds, while the methyl group directly bound to the amino moiety is stabilized inside the π basic cavity via cation⋯π inter-actions. The chloride counter-anion is located between the alkyl legs of the cavitand, forming C-H⋯Cl inter-actions with the aromatic and methyl-enic H atoms of the lower rim. The chloride anion is also responsible for the formation of a supra-molecular chain along the b-axis direction through C-H⋯Cl inter-actions involving the phenyl substituent of one phospho-nate group. C-H⋯O and C-H⋯π inter-actions between the guest and adjacent cavitands contribute to the formation of the crystal structure.
Keywords: crystal structure; illicit drugs; inclusion compounds; mephedrone; tetraphosphonate cavitands.
Figures
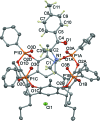
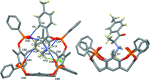

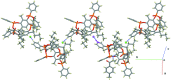
 + y,
+ y,  − z.
− z.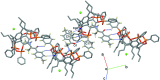
 + y,
+ y,  − z.
− z.

References
-
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst. 32, 115–119.
-
- Biavardi, E., Battistini, G., Montalti, M., Yebeutchou, R. M., Prodi, L. & Dalcanale, E. (2008). Chem. Commun. pp. 1638. - PubMed
-
- Biavardi, E., Federici, S., Tudisco, C., Menozzi, D., Massera, C., Sottini, A., Condorelli, G. G., Bergese, P. & Dalcanale, E. (2014). Angew. Chem. Int. Ed. 53, 9183–9188. - PubMed
-
- Biavardi, E., Ugozzoli, F. & Massera, C. (2015). Chem. Commun. 51, 3426–3429. - PubMed
-
- Bruker (2008). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
