Synthesis of Oxazinanones: Intramolecular Cyclization of Amino Acid-Derived Diazoketones via Silica-Supported HClO4 Catalysis
- PMID: 30800653
- PMCID: PMC6376066
- DOI: 10.3389/fchem.2019.00062
Synthesis of Oxazinanones: Intramolecular Cyclization of Amino Acid-Derived Diazoketones via Silica-Supported HClO4 Catalysis
Abstract
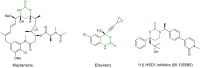
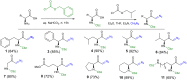
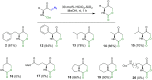
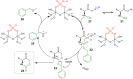
A Brønsted acid catalyzed intramolecular cyclization of N-Cbz-protected diazoketones, derived from α-amino acids, is described. The reaction proceeds under metal-free conditions and is promoted by ecofriendly silica-supported HClO4 as the catalyst and methanol as the solvent. This transformation enables the short synthesis of various 1,3-oxazinane-2,5-diones under mild reaction conditions and in good yields (up to 90%). The set-up is very simple; by just mixing all reagents together with no work-up necessary before purification, this protocol takes a greener approach.
Keywords: Brønsted acid; diazo carbonyls; heterocycles; intramolecular cyclization; oxazinanones.
Figures
References
-
- Bhanage B. M., Fujita S., Ikushima Y., Arai M. (2004). Non-catalytic clean synthesis route using urea to cyclic urea and cyclic urethane compounds. Green Chem. 6:78 10.1039/b310115k - DOI
LinkOut - more resources
Full Text Sources

