Pilot Study Examining Feasibility and Comparing the Effectiveness of Decision Aids for Hip and Knee Osteoarthritis: A Randomized Trial
- PMID: 30801033
- PMCID: PMC6378444
- DOI: 10.1177/2381468319827278
Pilot Study Examining Feasibility and Comparing the Effectiveness of Decision Aids for Hip and Knee Osteoarthritis: A Randomized Trial
Abstract
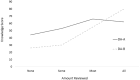
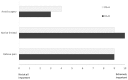
Background. There are many patient decision aids (DAs) available, yet there is limited evidence on comparative effectiveness of different tools. Objective. To examine feasibility of a study protocol and gather preliminary data on comparative effectiveness. Methods. Adult patients seeing a surgeon to discuss treatment for hip or knee osteoarthritis were randomized to hip and knee DAs from two vendors. Pre-visit survey included Hip/Knee Decision Quality Instrument, DA usage, health literacy, and quality of life (EQ-5D). Surgical status was ascertained 6 months post-visit. We examined response rates, eligibility, and compared the two DAs on amount of use, knowledge scores, and receipt of preferred treatment. Results. Overall response rate was 58/74 (78%) and did not differ by study arm. More patients in DA-A group reported reviewing all the DAs (64.5% DA-A v. 24.0% DA-B, P = 0.003). Knowledge scores were similar across arms (55.2% DA-A v. 48.8% DA-B, P = 0.4). For DA-B, knowledge scores were higher for those who reviewed all the DAs compared with those who did not (80% knowledge v. 39% knowledge, respectively, P = 0.004), while scores for DA-A did not vary by usage (62% knowledge v. 53% knowledge, respectively, P = 0.3). A similar percentage of each group received their preferred treatment (77% v. 73%, P = 0.8). Patients who were unsure about preferred treatment at baseline were more likely to have surgery in the DA-A arm compared with the DA-B arm (55% v. 20%, P = 0.1). Limitations. Small sample; patients were only surveyed pre-visit. Conclusion. Despite having different content and formats, the two DAs had similar overall effectiveness. Patients were more likely to review all of DA-A; however, patients who reviewed all of DA-B had the highest knowledge scores.
Keywords: hip osteoarthritis; knee osteoarthritis; patient decision aids; patient education; shared decision making.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Sepucha (PI) has received salary support as a medical editor for the Informed Medical Decisions Foundation (IMDF). From 1997 to 2014, the IMDF was associated with Health Dialog; from 2014 to 2017, the IMDF was part of Healthwise; and in 2017, the IMDF became part of Massachusetts General Hospital. Dr. Freiberg reports other support from Zimmer Biomet, ArthroSurface, CeramTec, and Orthopaedic Technology Group, outside the submitted work. Dr. Bedair reports personal fees from Smith & Nephew, personal fees from Conformis, outside the submitted work. Dr. Dwyer and Ms. Mangla declare that they have no competing interests.
Figures
References
-
- Joseph-Williams N, Newcombe R, Politi M, et al. Toward minimum standards for certifying patient decision aids: a modified Delphi consensus process. Med Decis Making. 2014;34(6):699–710. - PubMed
-
- Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134(8):663–94. - PubMed
-
- Hoffman AS, Sepucha KR, Abhyankar P, et al. Explanation and elaboration of the Standards for UNiversal reporting of patient Decision Aid Evaluations (SUNDAE) guidelines: examples of reporting SUNDAE items from patient decision aid evaluation literature. BMJ Qual Saf. 2018;27(5):389–412. - PMC - PubMed
LinkOut - more resources
Full Text Sources