A randomized open label trial of tamoxifen combined with amphotericin B and fluconazole for cryptococcal meningitis
- PMID: 30801037
- PMCID: PMC6381443
- DOI: 10.12688/wellcomeopenres.15010.1
A randomized open label trial of tamoxifen combined with amphotericin B and fluconazole for cryptococcal meningitis
Abstract
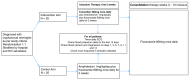
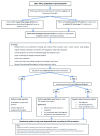
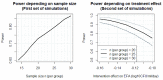
Background: Cryptococcal meningitis is a leading cause of death in HIV-infected patients. International treatment guidelines recommend induction therapy with amphotericin B and flucytosine. This antifungal combination is most effective, but unfortunately flucytosine is expensive and unavailable where the burden of disease is greatest. Where unavailable, guidelines recommend treatment with amphotericin and fluconazole, but this is less effective, with mortality rates of 40-50%. Faster rates of clearance of yeast from cerebrospinal fluid (CSF) are associated with better outcomes - improving the potency of antifungal therapy is likely to be an effective strategy to improve survival. Tamoxifen, a selective estrogen receptor modulator used to treat breast cancer, has anti-cryptococcal activity, appearing synergistic when combined in vitro with amphotericin, and fungicidal when combined with fluconazole. It is concentrated in the brain and macrophages, off-patent, cheap and widely available. We designed a randomized trial to deliver initial efficacy and safety data for tamoxifen combined with amphotericin and fluconazole. Method: A phase II, open-label, randomized (1:1) controlled trial of tamoxifen (300mg/day) combined with amphotericin (1mg/kg/day) and fluconazole (800mg/day) for the first 2 weeks therapy for HIV infected or uninfected adults with cryptococcal meningitis. The study recruits at Cho Ray Hospital and the Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam. The primary end point is Early Fungicidal Activity (EFA-the rate of yeast clearance from CSF), over the first two weeks of treatment. 50 patients will be recruited providing ≈80% and 90% power to detect a difference in the EFA of -0.11 or -0.13 log10CFU/ml/day, respectively. Discussion: The results of the study will inform the decision to proceed to a larger trial powered to mortality. The size of effect detectable has previously been associated with reduced mortality from this devastating disease. Particular side effects of interest include QT prolongation. Trial registration: Clinicaltrials.gov NCT03112031 (11/04/2017).
Keywords: Crytococcus; Tamoxifen; amphotericin B; antifungal therapy; cryptococcal meningitis; drug re-purposing; fluconazole.
Conflict of interest statement
No competing interests were disclosed.
Figures
References
-
- Day JN: Cryptococcal meningitis. Pract Neurol. 2004;4:274–285. 10.1111/j.1474-7766.2004.00254.x - DOI
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical