A Polyextreme Hydrothermal System Controlled by Iron: The Case of Dallol at the Afar Triangle
- PMID: 30801049
- PMCID: PMC6380227
- DOI: 10.1021/acsearthspacechem.8b00141
A Polyextreme Hydrothermal System Controlled by Iron: The Case of Dallol at the Afar Triangle
Abstract
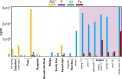
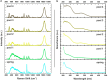
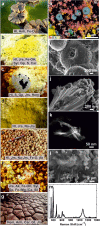
One of the latest volcanic features of the Erta Ale range at the Afar Triangle (NE Ethiopia) has created a polyextreme hydrothermal system located at the Danakil depression on top of a protovolcano known as the dome of Dallol. The interaction of the underlying basaltic magma with the evaporitic salts of the Danakil depression has generated a unique, high-temperature (108 °C), hypersaline (NaCl supersaturated), hyperacidic (pH values from 0.1 to -1.7), oxygen-free hydrothermal site containing up to 150 g/L of iron. We find that the colorful brine pools and mineral patterns of Dallol derive from the slow oxygen diffusion and progressive oxidation of the dissolved ferrous iron, the iron-chlorine/-sulfate complexation, and the evaporation. These inorganic processes induce the precipitation of nanoscale jarosite-group minerals and iron(III)-oxyhydroxides over a vast deposition of halite displaying complex architectures. Our results suggest that life, if present under such conditions, does not play a dominant role in the geochemical cycling and mineral precipitation at Dallol as opposed to other hydrothermal sites. Dallol, a hydrothermal system controlled by iron, is a present-day laboratory for studying the precipitation and progressive oxidation of iron minerals, relevant for geochemical processes occurring at early Earth and Martian environments.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Holwerda J. G.; Hutchinson R. W. Potash-bearing evaporites in the Danakil Region, Ethiopia. Econ. Geol. Bull. Soc. Econ. Geol. 1968, 63, 124–150. 10.2113/gsecongeo.63.2.124. - DOI
-
- Fazzini M.; Bisci C.. The climate of Ethiopia. In Landscapes and Landforms of Ethiopia; Billi P., Ed., World Geomorphological Landscapes; Springer, 2015.
-
- Berhanu B.; Seleshi Y.; Melesse A. M.. Surface water and groundwater resources of Ethiopia: Potentials and challenges of water resources development. In Nile River Basin, Ecohydrological Challenges, Climate Change and Hydropolitics; Melesse A. M.et al., Eds.; Springer, 2014.
-
- Makris J.; Ginzburg A. The Afar depression: transition between continental rifting and sea-floor spreading. Tectonophysics 1987, 141, 199–214. 10.1016/0040-1951(87)90186-7. - DOI
-
- Prodehl C. M.; et al. J. Crustal thinning in relationship to the evolution of the Afro-Arabian rift system: a review of seismic refraction data. Tectonophysics 1991, 198, 311–327. 10.1016/0040-1951(91)90158-O. - DOI
LinkOut - more resources
Full Text Sources
