New Developments in Imaging Idiopathic Pulmonary Fibrosis With Hyperpolarized Xenon Magnetic Resonance Imaging
- PMID: 30801449
- PMCID: PMC6392051
- DOI: 10.1097/RTI.0000000000000392
New Developments in Imaging Idiopathic Pulmonary Fibrosis With Hyperpolarized Xenon Magnetic Resonance Imaging
Abstract
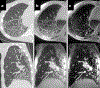
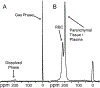
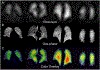
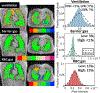
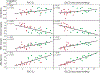
Idiopathic pulmonary fibrosis (IPF) is a progressive pulmonary disease that is ultimately fatal. Although the diagnosis of IPF has been revolutionized by high-resolution computed tomography, this imaging modality still exhibits significant limitations, particularly in assessing disease progression and therapy response. The need for noninvasive regional assessment has become more acute in light of recently introduced novel therapies and numerous others in the pipeline. Thus, it will likely be valuable to complement 3-dimensional imaging of lung structure with 3-dimensional regional assessment of function. This challenge is well addressed by hyperpolarized (HP) Xe magnetic resonance imaging (MRI), exploiting the unique properties of this inert gas to image its distribution, not only in the airspaces, but also in the interstitial barrier tissues and red blood cells. This single-breath imaging exam could ultimately become the ideal, noninvasive tool to assess pulmonary gas-exchange impairment in IPF. This review article will detail the evolution of HP Xe MRI from its early development to its current state as a clinical research platform. It will detail the key imaging biomarkers that can be generated from the Xe MRI examination, as well as their potential in IPF for diagnosis, prognosis, and assessment of therapeutic response. We conclude by discussing the types of studies that must be performed for HP Xe MRI to be incorporated into the IPF clinical algorithm and begin to positively impact IPF disease diagnosis and management.
Figures



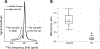
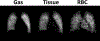






References
-
- Raghu G, Weycker D, Edelsberg J, Bradford WZ & Oster G Incidence and Prevalence of Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 174, 810–816 (2006). - PubMed
-
- Demedts M & Costabel U ATS/ERS international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Eur. Respir. J. 19, 794–796 (2002). - PubMed
-
- King TE et al. Idiopathic Pulmonary Fibrosis: Diagnosis and Treatment. Am. J. Respir. Crit. Care Med. 161, 646–664 (2000). - PubMed
-
- King TE, Pardo A & Selman M Idiopathic pulmonary fibrosis. Lancet 378, 1949–1961 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

