Effect of Timing and Complement Receptor Antagonism on Intragraft Recruitment and Protolerogenic Effects of Mesenchymal Stromal Cells in Murine Kidney Transplantation
- PMID: 30801518
- PMCID: PMC6934941
- DOI: 10.1097/TP.0000000000002611
Effect of Timing and Complement Receptor Antagonism on Intragraft Recruitment and Protolerogenic Effects of Mesenchymal Stromal Cells in Murine Kidney Transplantation
Abstract
Background: Mesenchymal stromal cells (MSCs) have protolerogenic effects in renal transplantation, but they induce long-term regulatory T cells (Treg)-dependent graft acceptance only when infused before transplantation. When given posttransplant, MSCs home to the graft where they promote engraftment syndrome and do not induce Treg. Unfortunately, pretransplant MSC administration is unfeasible in deceased-donor kidney transplantation.
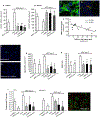
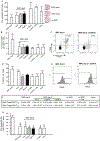
Methods: To make MSCs a therapeutic option also for deceased organ recipients, we tested whether MSC infusion at the time of transplant (day 0) or posttransplant (day 2) together with inhibition of complement receptors prevents engraftment syndrome and allows their homing to secondary lymphoid organs for promoting tolerance. We analyzed intragraft and splenic MSC localization, graft survival, and alloimmune response in mice recipients of kidney allografts and syngeneic MSCs given on day 0 or on posttransplant day 2. C3a receptor (C3aR) or C5a receptor (C5aR) antagonists were administered to mice in combination with the cells or were used together to treat MSCs before infusion.
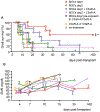
Results: Syngeneic MSCs given at day 0 homed to the spleen increased Treg numbers and induced long-term graft acceptance. Posttransplant MSC infusion, combined with a short course of C3aR or C5aR antagonist or administration of MSCs pretreated with C3aR and C5aR antagonists, prevented intragraft recruitment of MSCs and graft inflammation, inhibited antidonor T-cell reactivity, but failed to induce Treg, resulting in mild prolongation of graft survival.
Conclusions: These data support testing the safety/efficacy profile of administering MSCs on the day of transplant in deceased-donor transplant recipients and indicate that complement is crucial for MSC recruitment into the kidney allograft.
Conflict of interest statement
DISCLOSURE
The Authors declare no conflicts of interest.
Figures
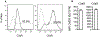
Similar articles
-
Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells.J Immunol. 2008 Sep 15;181(6):3933-46. doi: 10.4049/jimmunol.181.6.3933. J Immunol. 2008. PMID: 18768848
-
TGF-β1-Licensed Murine MSCs Show Superior Therapeutic Efficacy in Modulating Corneal Allograft Immune Rejection In Vivo.Mol Ther. 2020 Sep 2;28(9):2023-2043. doi: 10.1016/j.ymthe.2020.05.023. Epub 2020 May 30. Mol Ther. 2020. PMID: 32531237 Free PMC article.
-
Localization of mesenchymal stromal cells dictates their immune or proinflammatory effects in kidney transplantation.Am J Transplant. 2012 Sep;12(9):2373-83. doi: 10.1111/j.1600-6143.2012.04115.x. Epub 2012 May 29. Am J Transplant. 2012. PMID: 22642544
-
Mesenchymal stromal cells in renal transplantation: opportunities and challenges.Nat Rev Nephrol. 2016 Apr;12(4):241-53. doi: 10.1038/nrneph.2016.7. Epub 2016 Feb 8. Nat Rev Nephrol. 2016. PMID: 26853275 Review.
-
Mesenchymal stromal cells to promote solid organ transplantation tolerance.Curr Opin Organ Transplant. 2013 Feb;18(1):51-8. doi: 10.1097/MOT.0b013e32835c5016. Curr Opin Organ Transplant. 2013. PMID: 23254705 Review.
Cited by
-
Mesenchymal Stem Cell Utilization for In Vitro Donor Liver Machine Perfusion Preservation: Current Status and Future Directions.Stem Cells Transl Med. 2023 Oct 5;12(10):665-675. doi: 10.1093/stcltm/szad053. Stem Cells Transl Med. 2023. PMID: 37643740 Free PMC article. Review.
-
When Origin Matters: Properties of Mesenchymal Stromal Cells From Different Sources for Clinical Translation in Kidney Disease.Front Med (Lausanne). 2021 Sep 20;8:728496. doi: 10.3389/fmed.2021.728496. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34616756 Free PMC article. Review.
-
Improved transduction of canine X-linked muscular dystrophy with rAAV9-microdystrophin via multipotent MSC pretreatment.Mol Ther Methods Clin Dev. 2020 Nov 17;20:133-141. doi: 10.1016/j.omtm.2020.11.003. eCollection 2021 Mar 12. Mol Ther Methods Clin Dev. 2020. PMID: 33426145 Free PMC article.
-
Donor adipose-derived stromal cells are vasoprotectant but unable to revert acute rejection in rodent vascularized composite allotransplants.Front Immunol. 2025 Apr 28;16:1581599. doi: 10.3389/fimmu.2025.1581599. eCollection 2025. Front Immunol. 2025. PMID: 40356930 Free PMC article.
-
Autologous Mesenchymal Stem Cells for Treatment of Chronic Active Antibody-Mediated Kidney Graft Rejection: Report of the Phase I/II Clinical Trial Case Series.Transpl Int. 2022 Nov 22;35:10772. doi: 10.3389/ti.2022.10772. eCollection 2022. Transpl Int. 2022. PMID: 36484064 Free PMC article. Clinical Trial.
References
-
- Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–396. - PubMed
-
- Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107(4):1484–1490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

