Treatment of melanoma of unknown primary in the era of immunotherapy and targeted therapy: A Dutch population-based study
- PMID: 30801710
- PMCID: PMC6900034
- DOI: 10.1002/ijc.32229
Treatment of melanoma of unknown primary in the era of immunotherapy and targeted therapy: A Dutch population-based study
Abstract
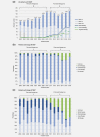
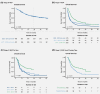
Melanoma of unknown primary (MUP) may have a different biology to melanoma of known primary, but clinical trials of novel therapies (e.g., immune checkpoint or BRAF/MEK inhibitors) have not reported the outcomes in this population. We therefore evaluated the overall survival (OS) among patients with MUP in the era of novel therapy. Data for stage III or IV MUP were extracted from a nationwide database for the period 2003-2016, with classification based on the eighth edition of the American Joint Committee on Cancer criteria. The population was divided into pre- (2003-2010) and post- (2011-2016) novel therapy eras. Also, OS in the post-novel era was compared between patients with stage IV MUP by whether they received novel therapy. In total, 2028 of 65,110 patients (3.1%) were diagnosed with MUP. Metastatic sites were known in 1919 of 2028 patients, and most had stage IV disease (53.8%). For patients with stage III MUP, the 5-year OS rates were 48.5% and 50.2% in the pre- and post-novel eras, respectively (p = 0.948). For those with stage IV MUP, the median OS durations were unchanged in the pre-novel era and post-novel era when novel therapy was not used (both 4 months); however, OS improved to 11 months when novel therapy was used in the post-novel era (p < 0.001). In conclusion, more than half of the patients with MUP are diagnosed with stage IV and the introduction of novel therapy appears to have significantly improved the OS of these patients.
Keywords: immunotherapy; melanoma; targeted therapy; unknown primary.
© 2019 The Authors. International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Figures
Comment in
-
Author's reply to: The real-world outcome of metastatic melanoma: Unknown primary vs. known cutaneous.Int J Cancer. 2019 Dec 1;145(11):3175-3176. doi: 10.1002/ijc.32630. Epub 2019 Sep 3. Int J Cancer. 2019. PMID: 31423580 No abstract available.
-
The real-world outcome of metastatic melanoma: Unknown primary vs. known cutaneous.Int J Cancer. 2019 Dec 1;145(11):3173-3174. doi: 10.1002/ijc.32631. Epub 2019 Sep 3. Int J Cancer. 2019. PMID: 31423583 No abstract available.
-
Author's reply to: Air pollution and incident bladder cancer: A risk assessment.Int J Cancer. 2019 Dec 1;145(11):3178. doi: 10.1002/ijc.32632. Epub 2019 Aug 30. Int J Cancer. 2019. PMID: 31423586 No abstract available.
Similar articles
-
Clinical outcome of patients with metastatic melanoma of unknown primary in the era of novel therapy.Cancer Immunol Immunother. 2021 Nov;70(11):3123-3135. doi: 10.1007/s00262-021-02871-1. Epub 2021 Mar 27. Cancer Immunol Immunother. 2021. PMID: 33774697 Free PMC article.
-
Treatment Outcomes for Metastatic Melanoma of Unknown Primary in the New Era: A Single-Institution Study and Review of the Literature.Oncology. 2017;93(4):249-258. doi: 10.1159/000478050. Epub 2017 Jul 27. Oncology. 2017. PMID: 28746931 Free PMC article. Review.
-
Melanoma of unknown primary: New perspectives for an old story.Crit Rev Oncol Hematol. 2021 Feb;158:103208. doi: 10.1016/j.critrevonc.2020.103208. Epub 2020 Dec 28. Crit Rev Oncol Hematol. 2021. PMID: 33383207 Review.
-
Melanoma of unknown primary origin: a population-based study in the Netherlands.Eur J Cancer. 2013 Feb;49(3):676-83. doi: 10.1016/j.ejca.2012.09.005. Epub 2012 Sep 29. Eur J Cancer. 2013. PMID: 23031553
-
Improved survival for stage IV melanoma from an unknown primary site.J Clin Oncol. 2009 Jul 20;27(21):3489-95. doi: 10.1200/JCO.2008.18.9845. Epub 2009 May 18. J Clin Oncol. 2009. PMID: 19451446 Free PMC article.
Cited by
-
The Diminishing Importance of Primary Site Identification in Cancer of Unknown Primary: A Canadian Single-Center Experience.Front Oncol. 2021 Mar 3;11:634563. doi: 10.3389/fonc.2021.634563. eCollection 2021. Front Oncol. 2021. PMID: 33747958 Free PMC article.
-
Melanoma of Unknown Primary: Evaluation of the Characteristics, Treatment Strategies, Prognostic Factors in a Monocentric Retrospective Study.Front Oncol. 2021 Mar 5;11:627527. doi: 10.3389/fonc.2021.627527. eCollection 2021. Front Oncol. 2021. PMID: 33747946 Free PMC article.
-
Uncommon Subtypes of Malignant Melanomas: A Review Based on Clinical and Molecular Perspectives.Cancers (Basel). 2020 Aug 21;12(9):2362. doi: 10.3390/cancers12092362. Cancers (Basel). 2020. PMID: 32825562 Free PMC article. Review.
-
Canine melanoma: A review of diagnostics and comparative mechanisms of disease and immunotolerance in the era of the immunotherapies.Front Vet Sci. 2023 Jan 6;9:1046636. doi: 10.3389/fvets.2022.1046636. eCollection 2022. Front Vet Sci. 2023. PMID: 36686160 Free PMC article. Review.
-
Construction, validation and, visualization of a web-based nomogram to identify the best candidates for primary tumor resection in advanced cutaneous melanoma patients.Front Surg. 2023 Jan 18;9:975690. doi: 10.3389/fsurg.2022.975690. eCollection 2022. Front Surg. 2023. PMID: 36743900 Free PMC article.
References
-
- Sacchetto L, Zanetti R, Comber H, et al. Trends in incidence of thick, thin and in situ melanoma in Europe. Eur J Cancer 2018;92:108–18. - PubMed
-
- Kamposioras K, Pentheroudakis G, Pectasides D, et al. Malignant melanoma of unknown primary site. To make the long story short. A systematic review of the literature. Crit Rev Oncol Hematol 2011;78:112–26. - PubMed
-
- de Waal AC, Aben KK, van Rossum MM, et al. Melanoma of unknown primary origin: a population‐based study in The Netherlands. Eur J Cancer 2013;49:676–83. - PubMed
-
- Bae JM, Choi YY, Kim DS, et al. Metastatic melanomas of unknown primary show better prognosis than those of known primary: a systematic review and meta‐analysis of observational studies. J Am Acad Dermatol 2015;72:59–70. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

