Gi/o protein-coupled receptors inhibit neurons but activate astrocytes and stimulate gliotransmission
- PMID: 30801845
- PMCID: PMC6462242
- DOI: 10.1002/glia.23589
Gi/o protein-coupled receptors inhibit neurons but activate astrocytes and stimulate gliotransmission
Abstract
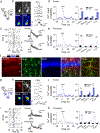
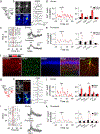
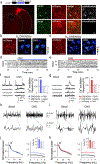
G protein-coupled receptors (GPCRs) play key roles in intercellular signaling in the brain. Their effects on cellular function have been largely studied in neurons, but their functional consequences on astrocytes are less known. Using both endogenous and chemogenetic approaches with DREADDs, we have investigated the effects of Gq and Gi/o GPCR activation on astroglial Ca2+ -based activity, gliotransmitter release, and the functional consequences on neuronal electrical activity. We found that while Gq GPCR activation led to cellular activation in both neurons and astrocytes, Gi/o GPCR activation led to cellular inhibition in neurons and cellular activation in astrocytes. Astroglial activation by either Gq or Gi/o protein-mediated signaling stimulated gliotransmitter release, which increased neuronal excitability. Additionally, activation of Gq and Gi/o DREADDs in vivo increased astrocyte Ca2+ activity and modified neuronal network electrical activity. Present results reveal additional complexity of the signaling consequences of excitatory and inhibitory neurotransmitters in astroglia-neuron network operation and brain function.
Keywords: DREADDs; G protein-coupled receptors; astrocyte Ca2+; astrocytes; gliotransmission.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures





References
-
- Agarwal A, Wu PH, Hughes EG, Fukaya M, Tischfield MA, Langseth AJ, . . . Bergles DE (2017). Transient Opening of the Mitochondrial Permeability Transition Pore Induces Microdomain Calcium Transients in Astrocyte Processes. Neuron, 93(3), 587-605 e587. doi: 10.1016/j.neuron.2016.12.034 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

