Therapeutic efficacy evaluation of radioimmunotherapy with 90 Y-labeled anti-podoplanin antibody NZ-12 for mesothelioma
- PMID: 30801908
- PMCID: PMC6500970
- DOI: 10.1111/cas.13979
Therapeutic efficacy evaluation of radioimmunotherapy with 90 Y-labeled anti-podoplanin antibody NZ-12 for mesothelioma
Abstract
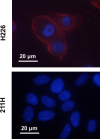
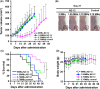
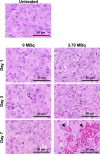
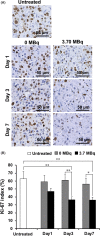
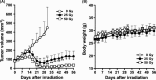
Podoplanin is a type I transmembrane sialomucin-like glycoprotein that is highly expressed in malignant mesothelioma. The rat-human chimeric antibody NZ-12 has high affinity for human podoplanin and antibody-dependent cellular cytotoxicity and is applicable for radioimmunotherapy (RIT) to enhance the antitumor effect. In the present study, we evaluated the in vivo and in vitro properties of radiolabeled NZ-12 and the antitumor effect of RIT with 90 Y-labeled NZ-12 in an NCI-H226 (H226) malignant mesothelioma xenograft mouse model. 111 In-labeled NZ-12 bound specifically to H226 cells with high affinity, and accumulation was high in H226 tumors but low in major organs. RIT with 90 Y-labeled NZ-12 significantly suppressed tumor growth and prolonged survival without body weight loss and obvious adverse effects. Higher podoplanin expression levels were observed in human mesothelioma specimens, suggesting higher tumor accumulation of 90 Y-labeled NZ-12 in patients compared with the H226 tumor xenografts. Our findings suggest that 90 Y-labeled NZ-12 is a promising RIT agent as a new therapeutic option for malignant mesothelioma that warrants further clinical studies to evaluate the dosimetry and efficacy in patients.
Keywords: antibody; malignant mesothelioma; podoplanin; radiation; radioimmunotherapy.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Authors declare no conflicts of interest for this article.
Figures


References
-
- Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353(15):1591‐1603. - PubMed
-
- Sterman DH, Albelda SM. Advances in the diagnosis, evaluation, and management of malignant pleural mesothelioma. Respirology. 2005;10(3):266‐283. - PubMed
-
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636‐2644. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

