Genetically heterogeneous mice exhibit a female survival advantage that is age- and site-specific: Results from a large multi-site study
- PMID: 30801953
- PMCID: PMC6516160
- DOI: 10.1111/acel.12905
Genetically heterogeneous mice exhibit a female survival advantage that is age- and site-specific: Results from a large multi-site study
Abstract
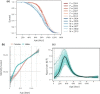
The female survival advantage is a robust characteristic of human longevity. However, underlying mechanisms are not understood, and rodent models exhibiting a female advantage are lacking. Here, we report that the genetically heterogeneous (UM-HET3) mice used by the National Institute on Aging Interventions Testing Program (ITP) are such a model. Analysis of age-specific survival of 3,690 control ITP mice revealed a female survival advantage paralleling that of humans. As in humans, the female advantage in mice was greatest in early adulthood, peaking around 350 days of age and diminishing progressively thereafter. This persistent finding was observed at three geographically distinct sites and in six separate cohorts over a 10-year period. Because males weigh more than females and bodyweight is often inversely related to lifespan, we examined sex differences in the relationship between bodyweight and survival. Although present in both sexes, the inverse relationship between bodyweight and longevity was much stronger in males, indicating that male mortality is more influenced by bodyweight than is female mortality. In addition, male survival varied more across site and cohort than female survival, suggesting greater resistance of females to environmental modulators of survival. Notably, at 24 months the relationship between bodyweight and longevity shifted from negative to positive in both sexes, similar to the human condition in advanced age. These results indicate that the UM-HET3 mouse models the human female survival advantage and provide evidence for greater resilience of females to modulators of survival.
Keywords: age-specific mortality; gender differences; lifespan; sex differences; smoothed hazard estimation; somatotrophic axis.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures


References
-
- Austad, S. N. (2011). Sex Differences in Longevity and Aging In Edward M., & Austad S. (Eds.), Handbook of the Biology of Aging, (7th ed., pp. 479–496). London: Academic Press.
-
- Bartke, A. , Heiman, M. , Turyn, D. , Dominici, F. , & Kopchick, J. J. (2004). The role of growth hormone signaling in the control of ageing. NeuroImmune Biology, 4, 123–137. 10.1177/1073858411407206.Epub. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

