Electrical synapses regulate both subthreshold integration and population activity of principal cells in response to transient inputs within canonical feedforward circuits
- PMID: 30802238
- PMCID: PMC6405166
- DOI: 10.1371/journal.pcbi.1006440
Electrical synapses regulate both subthreshold integration and population activity of principal cells in response to transient inputs within canonical feedforward circuits
Abstract
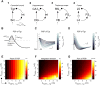
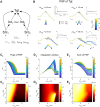
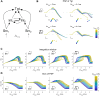
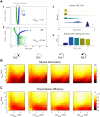
As information about the world traverses the brain, the signals exchanged between neurons are passed and modulated by synapses, or specialized contacts between neurons. While neurotransmitter-based synapses tend to exert either excitatory or inhibitory pulses of influence on the postsynaptic neuron, electrical synapses, composed of plaques of gap junction channels, continuously transmit signals that can either excite or inhibit a coupled neighbor. A growing body of evidence indicates that electrical synapses, similar to their chemical counterparts, are modified in strength during physiological neuronal activity. The synchronizing role of electrical synapses in neuronal oscillations has been well established, but their impact on transient signal processing in the brain is much less understood. Here we constructed computational models based on the canonical feedforward neuronal circuit and included electrical synapses between inhibitory interneurons. We provided discrete closely-timed inputs to the circuits, and characterize the influence of electrical synapse strength on both subthreshold summation and spike trains in the output neuron. Our simulations highlight the diverse and powerful roles that electrical synapses play even in simple circuits. Because these canonical circuits are represented widely throughout the brain, we expect that these are general principles for the influence of electrical synapses on transient signal processing across the brain.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron. 2004;41(4):495–511. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

