In vitro activity and mode of action of phenolic compounds on Leishmania donovani
- PMID: 30802252
- PMCID: PMC6405172
- DOI: 10.1371/journal.pntd.0007206
In vitro activity and mode of action of phenolic compounds on Leishmania donovani
Abstract
Background: Leishmaniasis is a disease caused by the protozoan parasite, Leishmania. The disease remains a global threat to public health requiring effective chemotherapy for control and treatment. In this study, the effect of some selected phenolic compounds on Leishmania donovani was investigated. The compounds were screened for their anti-leishmanial activities against promastigote and intracellular amastigote forms of Leishmania donovani.
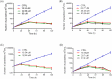
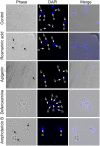
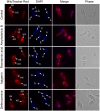
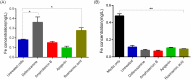
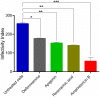
Methodology/principal findings: The dose dependent effect and cytotoxicity of the compounds were determined by the MTT assay. Flow cytometry was used to determine the effect of the compounds on the cell cycle. Parasite morphological analysis was done by microscopy and growth kinetic studies were conducted by culturing cells and counting at 24 hours intervals over 120 hours. The cellular levels of iron in promastigotes treated with compounds was determined by atomic absorption spectroscopy and the effect of compounds on the expression of iron dependent enzymes was investigated using RT-qPCR. The IC50 of the compounds ranged from 16.34 μM to 198 μM compared to amphotericin B and deferoxamine controls. Rosmarinic acid and apigenin were the most effective against the promastigote and the intracellular amastigote forms. Selectivity indexes (SI) of rosmarinic acid and apigenin were 15.03 and 10.45 respectively for promastigotes while the SI of 12.70 and 5.21 respectively was obtained for intracellular amastigotes. Morphologically, 70% of rosmarinic acid treated promastigotes showed rounded morphology similar to the deferoxamine control. About 30% of cells treated with apigenin showed distorted cell membrane. Rosmarinic acid and apigenin induced cell arrest in the G0/G1 phase in promastigotes. Elevated intracellular iron levels were observed in promastigotes when parasites were treated with rosmarinic acid and this correlated with the level of expression of iron dependent genes.
Conclusions/significance: The data suggests that rosmarinic acid exerts its anti-leishmanial effect via iron chelation resulting in variable morphological changes and cell cycle arrest.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Das VN, Pandey RN, Siddiqui NA, Chapman LA, Kumar V, Pandey K, et al. Longitudinal Study of Transmission in Households with Visceral Leishmaniasis, Asymptomatic Infections and PKDL in Highly Endemic Villages in Bihar, India. PLoS Negl Trop Dis. 2016;10(12):e0005196 10.1371/journal.pntd.0005196 - DOI - PMC - PubMed
-
- Croft SL, Coombs GH. Leishmaniasis--;current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003;19(11):502–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

