Primary HIV prevention in pregnant and lactating Ugandan women: A randomized trial
- PMID: 30802277
- PMCID: PMC6388930
- DOI: 10.1371/journal.pone.0212119
Primary HIV prevention in pregnant and lactating Ugandan women: A randomized trial
Abstract
Background: The 'Primary HIV Prevention among Pregnant and Lactating Ugandan Women' (PRIMAL) study aimed to assess the effectiveness of an enhanced HIV counseling intervention for preventing HIV acquisition among HIV-uninfected mothers during pregnancy and throughout the breastfeeding period.
Methods: We conducted an unblinded randomized control trial between 22 February 2013 and 22 April 2016 to assess the effectiveness of an extended repeat HIV testing and enhanced counseling (ERHTEC) intervention aimed at preventing primary HIV infection among HIV-uninfected pregnant and lactating women in Uganda. HIV-uninfected pregnant women aged 15-49 were enrolled 1:1 individually or in couples together with their partner. Enrolled women and couples were randomized 1:1 to an intervention (ERHTEC) or control (extended repeat HIV testing and standard counseling) group and followed up to 24 months postpartum or six weeks past complete cessation of breastfeeding, whichever came first. Both groups were tested for sexually transmitted infections (STIs) and HIV at enrollment, delivery, 3 and 6 months postpartum and every 6 months thereafter until the end of follow-up. The intervention group received enhanced HIV prevention counseling every 3 months throughout follow-up. The control group received standard counseling at the time of HIV retesting. Both intervention and control couples were offered couple HIV testing and counseling at all study visits.
Main outcome measures: Frequency of condom use and incidence of HIV, syphilis, gonorrhea, chlamydia and trichomoniasis over follow-up.
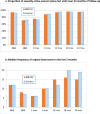
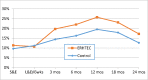
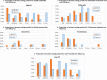
Results: Between February 2013 and April 2014, we enrolled 820 HIV-uninfected pregnant women presenting for antenatal care individually (n = 410) or in couples (n = 410 women and 410 partners) in one urban and one rural public Ugandan hospital. Women's median age was 24 years (IQR 20-28 years). At baseline, participants did not differ in any socio-demographic, reproductive health, HIV testing history, sexual behavior, medical history or STI status characteristics; 96% (386/402) of couples were tested and counseled for HIV together with their partners at enrolment, 2.1% (7/329) of whom were found to be HIV-infected. Six hundred twenty-five (76%) women completed follow-up as per protocol (S1 Protocol). Women were followed for an average of 1.76 years and cumulated 1,439 women-years of follow-up or 81% of the maximum 1,779 women-years of follow-up assuming no dropouts. Men were followed for an average of 1.72 years. The frequency of consistent condom use and the proportion of women who used condoms over the last 3 months or at last vaginal sex increased substantially over follow-up in both arms, but there were no statistically significant differences in increases between the intervention and control arms. During follow-up, on average 42% (range 36%-46%) of couple partners were counseled together. Between 3.8% and 7.6% of women tested positive at any follow-up visit for any STI including syphilis, gonorrhea, C. trachomatis or T. vaginalis. Four women (two in each arm) and no enrolled men became infected with HIV, representing an overall HIV incidence rate of 0.186 per 100 person-years. Three of the women seroconverters had enrolled individually, one as a couple. At or before seroconversion, all four women reported their partners had extramarital relationships and/or had not disclosed their suspected HIV-infected status. There were no statistically significant differences between study arms for STI or HIV incidences.
Conclusions: A sustained enhanced HIV prevention counseling intervention for up to 2 years postpartum among pregnant and breastfeeding women did not have a statistically significant effect on condom use or HIV incidence among these women. However, in both study arms, condom use increased over follow-up while STI and HIV incidence remained very low when compared to similar cohorts in and outside Uganda, suggesting that repeat HIV testing during breastfeeding, whether with enhanced or standard counseling, may have had an unintended HIV preventive effect among pregnant and lactating women in this setting. Further research is needed to verify this hypothesis.
Trial registration: ClinicalTrials.gov NCT01882998.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- UNAIDS. Ending AIDS: Progress towards the 90-90-90 targets'. Geneva, Switzerland: 2017.
-
- WHO. PMTCT strategic vision 2010–2015: Preventing mother-to-child transmission of HIV to reach the UNGASS and Millennium Development Goals. World Health Organization, 20 Av. Appia, Geneva, Switzerland, 2010. Contract No.: ISBN 978 92 4 159903 0.
-
- UNAIDS. The Global Plan Towards the Elimination of New HIV Infections Among Children by 2015. Geneva, Switzerland: UNAIDS, 2015.

