Fibroblast Growth Factor-21 Controls Dietary Protein Intake in Male Mice
- PMID: 30802283
- PMCID: PMC6469953
- DOI: 10.1210/en.2018-01056
Fibroblast Growth Factor-21 Controls Dietary Protein Intake in Male Mice
Abstract
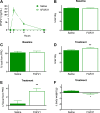
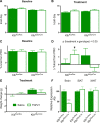
Whereas carbohydrates and lipids are stored as glycogen and fat, there is no analogous inert storage form of protein. Therefore, continuous adjustments in feeding behavior are needed to match amino acid supply to ongoing physiologic need. Neuroendocrine mechanisms facilitating this behavioral control of protein and amino acid homeostasis remain unclear. The hepatokine fibroblast growth factor-21 (FGF21) is well positioned for such a role, as it is robustly secreted in response to protein and/or amino acid deficit. In this study, we tested the hypothesis that FGF21 feeds back at its receptors in the nervous system to shift macronutrient selection toward protein. In a series of behavioral tests, we isolated the effect of FGF21 to influence consumption of protein, fat, and carbohydrate in male mice. First, we used a three-choice pure macronutrient-diet paradigm. In response to FGF21, mice increased consumption of protein while reducing carbohydrate intake, with no effect on fat intake. Next, to determine whether protein or carbohydrate was the primary-regulated nutrient, we used a sequence of two-choice experiments to isolate the effect of FGF21 on preference for each macronutrient. Sweetness was well controlled by holding sucrose constant across the diets. Under these conditions, FGF21 increased protein intake, and this was offset by reducing the consumption of either carbohydrate or fat. When protein was held constant, FGF21 had no effect on macronutrient intake. Lastly, the effect of FGF21 to increase protein intake required the presence of its co-receptor, β-klotho, in neurons. Taken together, these findings point to a novel liver→nervous system pathway underlying the regulation of dietary protein intake via FGF21.
Copyright © 2019 Endocrine Society.
Figures




References
-
- Solon-Biet SM, Cogger VC, Pulpitel T, Heblinski M, Wahl D, McMahon AC, Warren A, Durrant-Whyte J, Walters KA, Krycer JR, Ponton F, Gokarn R, Wali JA, Ruohonen K, Conigrave AD, James DE, Raubenheimer D, Morrison CD, Le Couteur DG, Simpson SJ. Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metab. 2016;24(4):555–565. - PubMed
-
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19(3):418–430. - PMC - PubMed
-
- Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492(1):203–206. - PubMed
-
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–1635. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

