Pharmacokinetics of 38% topical silver diamine fluoride in healthy adult volunteers
- PMID: 30803490
- PMCID: PMC6936220
- DOI: 10.1016/j.adaj.2018.10.018
Pharmacokinetics of 38% topical silver diamine fluoride in healthy adult volunteers
Abstract
Background: Silver diamine fluoride (SDF) is used topically to prevent or arrest caries. The authors' aim was to characterize the kinetics of silver and fluoride after topical application of SDF.
Methods: Sixteen adults participated in a pharmacokinetics study after the application of 38% SDF to 5 teeth (approximately 50 microliters, estimated 4-11 milligrams per participant). Serum and urine samples were collected over 24 hours after application and were analyzed for silver and fluoride.
Results: Silver serum peak was 0.67 (standard deviation [SD], 0.49) nanograms per milliliter; median time to peak was 3 hours. The estimated elimination half-life of silver was 46 (SD, 26) hours. No silver was recovered in urine. Baseline fluoride serum levels ranged from less than 10 through 50 ng/mL (< 0.01-0.05 parts per million) and fluctuated around baseline after SDF. The 24-hour urinary fluoride was 1.29 (SD, 0.81) mg.
Conclusions: SDF was well tolerated in this study, and no adverse events related to SDF were reported.
Practical implications: This clinical study confirmed that topical application of 38% SDF, in growing use in the United States, is safe and well tolerated in healthy adults.
Keywords: Silver diamine fluoride; clinical study; dental; healthy volunteers.
Copyright © 2019 American Dental Association. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Conflicting Interests:
PM is a director of Advantage Silver Dental Arrest, LLC. The other authors declare no conflict of interest.
Figures
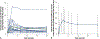

References
-
- Slayton RL, Urquhart O, Araujo MWB, et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions: A report from the American Dental Association. JADA. 2018;149(10):837–849.e19. - PubMed
-
- Craig GG, Knight GM, McIntyre JM. Clinical evaluation of diamine silver fluoride/potassium iodide as a dentine desensitizing agent. A pilot study. Aust Dent J. 2012;57:308–311. - PubMed
-
- Tan HP, Lo EC, Dyson JE, et al. A randomized trial on root caries prevention in elders. J Dent Res. 2010;89:1086–1090. - PubMed
-
- Zhang W, McGrath C, Lo EC, et al. Silver diamine fluoride and education to prevent and arrest root caries among community-dwelling elders. Caries Res. 2013;47:284–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

