mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases
- PMID: 30803823
- PMCID: PMC6453507
- DOI: 10.1016/j.ymthe.2019.01.020
mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases
Abstract
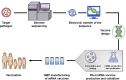
In the last two decades, there has been growing interest in mRNA-based technology for the development of prophylactic vaccines against infectious diseases. Technological advancements in RNA biology, chemistry, stability, and delivery systems have accelerated the development of fully synthetic mRNA vaccines. Potent, long-lasting, and safe immune responses observed in animal models, as well as encouraging data from early human clinical trials, make mRNA-based vaccination an attractive alternative to conventional vaccine approaches. Thanks to these data, together with the potential for generic, low-cost manufacturing processes and the completely synthetic nature, the prospects for mRNA vaccines are very promising. In addition, mRNA vaccines have the potential to streamline vaccine discovery and development, and facilitate a rapid response to emerging infectious diseases. In this review, we overview the unique attributes of mRNA vaccine approaches, review the data of mRNA vaccines against infectious diseases, discuss the current challenges, and highlight perspectives about the future of this promising technology.
Keywords: RNA-based vaccine; infectious diseases; self-amplifying mRNA; synthetic vaccine; vaccine on demand.
Copyright © 2019 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Jenner E. Sampson Low; 1978. An Inquiry into the Causes and Effects of the Variolae Vaccinae, a Disease Discovered in Some of the Western Counties of England, Particularly Gloucestershire, and Known by the Name of the Cow Pox.
-
- Younger D.S., Younger A.P., Guttmacher S. Childhood vaccination: implications for global and domestic public health. Neurol. Clin. 2016;34:1035–1047. - PubMed
-
- Plotkin S.A., Plotkin S.L. The development of vaccines: how the past led to the future. Nat. Rev. Microbiol. 2011;9:889–893. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

