Potentiation of complement regulator factor H protects human endothelial cells from complement attack in aHUS sera
- PMID: 30804016
- PMCID: PMC6391659
- DOI: 10.1182/bloodadvances.2018025692
Potentiation of complement regulator factor H protects human endothelial cells from complement attack in aHUS sera
Abstract
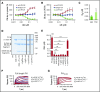
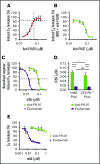
Mutations in the gene encoding for complement regulator factor H (FH) severely disrupt its normal function to protect human cells from unwanted complement activation, resulting in diseases such as atypical hemolytic uremic syndrome (aHUS). aHUS presents with severe hemolytic anemia, thrombocytopenia, and renal disease, leading to end-stage renal failure. Treatment of severe complement-mediated disease, such as aHUS, by inhibiting the terminal complement pathway, has proven to be successful but at the same time fails to preserve the protective role of complement against pathogens. To improve complement regulation on human cells without interfering with antimicrobial activity, we identified an anti-FH monoclonal antibody (mAb) that induced increased FH-mediated protection of primary human endothelial cells from complement, while preserving the complement-mediated killing of bacteria. Moreover, this FH-activating mAb restored complement regulation in sera from aHUS patients carrying various heterozygous mutations in FH known to impair FH function and dysregulate complement activation. Our data suggest that FH normally circulates in a less active conformation and can become more active, allowing enhanced complement regulation on human cells. Antibody-mediated potentiation of FH may serve as a highly effective approach to inhibit unwanted complement activation on human cells in a wide range of hematological diseases while preserving the protective role of complement against pathogens.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: R.B.P., M.C.B., T.W.K., and D.W. are coinventors of a patent (PCT/NL2015/050584) describing the potentiation of FH with mAbs and therapeutic uses thereof, which is licensed to Gemini Therapeutics, which provided partial financial support for this study. P.S.-C. has received speaking fees from Alexion Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures





References
-
- DiScipio RG. Ultrastructures and interactions of complement factors H and I. J Immunol. 1992;149(8):2592-2599. - PubMed
-
- Aslam M, Perkins SJ. Folded-back solution structure of monomeric factor H of human complement by synchrotron X-ray and neutron scattering, analytical ultracentrifugation and constrained molecular modelling. J Mol Biol. 2001;309(5):1117-1138. - PubMed
-
- Okemefuna AI, Nan R, Gor J, Perkins SJ. Electrostatic interactions contribute to the folded-back conformation of wild type human factor H. J Mol Biol. 2009;391(1):98-118. - PubMed
-
- Okemefuna AI, Gilbert HE, Griggs KM, Ormsby RJ, Gordon DL, Perkins SJ. The regulatory SCR-1/5 and cell surface-binding SCR-16/20 fragments of factor H reveal partially folded-back solution structures and different self-associative properties. J Mol Biol. 2008;375(1):80-101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

