In vitro and in vivo efficacy of an anti-CD203c conjugated antibody (AGS-16C3F) in mouse models of advanced systemic mastocytosis
- PMID: 30804017
- PMCID: PMC6391676
- DOI: 10.1182/bloodadvances.2018026179
In vitro and in vivo efficacy of an anti-CD203c conjugated antibody (AGS-16C3F) in mouse models of advanced systemic mastocytosis
Abstract
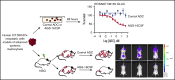
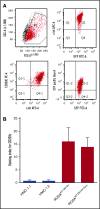
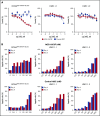
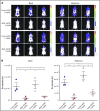
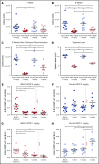
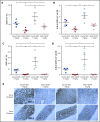
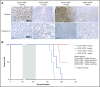
Antibody-drug conjugates (ADCs) are a new class of therapeutics that use antibodies to deliver potent cytotoxic drugs selectively to cancer cells. CD203c, an ecto-nucleotide pyrophosphatase-phosphodiesterase 3, is overexpressed on neoplastic mast cells (MCs) in systemic mastocytosis (SM), thus representing a promising target for antibody-mediated therapy. In this study, we have found that human neoplastic MC lines (ROSAKIT D816V and ROSAKIT D816V-Gluc), which express high levels of CD203c, are highly and specifically sensitive to the antiproliferative effects of an ADC against CD203c (AGS-16C3F). In these cell lines, AGS-16C3F induced cell apoptosis at very low concentrations. To characterize the effects of AGS-16C3F on leukemia progression in vivo, ROSAKIT D816V-Gluc NOD-SCID γ mouse models of advanced SM (AdvSM) were treated with AGS-16C3F or an ADC control for 2 weeks. Whereas AGS-16C3F had no apparent toxicity in xenotransplanted mice, in vivo neoplastic MC burden significantly decreased in both hematopoietic and nonhematopoietic organs. Furthermore, animals treated with AGS-16C3F had prolonged survival compared with the animals treated with control ADC, and AGS-16C3F efficiently prevented disease relapse. In conclusion, these preclinical studies identified CD203c as a novel therapeutic target on neoplastic MCs, and AGS-16C3F as a promising ADC for the treatment of patients with AdvSM.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: At the time of the research, H.K.-M. and F.D. were both employees of Agensys, Inc. The remaining authors declare no competing financial interests.
Figures
References
-
- Ustun C, Arock M, Kluin-Nelemans HC, et al. Advanced systemic mastocytosis: from molecular and genetic progress to clinical practice. Haematologica. 2016;101(10):1133-1143. - PubMed
-
- Valent P, Escribano L, Broesby-Olsen S, et al. ; European Competence Network on Mastocytosis. Proposed diagnostic algorithm for patients with suspected mastocytosis: a proposal of the European Competence Network on Mastocytosis. Allergy. 2014;69(10):1267-1274. - PubMed
-
- Arock M, Akin C, Hermine O, Valent P. Current treatment options in patients with mastocytosis: status in 2015 and future perspectives. Eur J Haematol. 2015;94(6):474-490. - PubMed
-
- Pardanani A. Systemic mastocytosis in adults: 2017 update on diagnosis, risk stratification and management. Am J Hematol. 2016;91(11):1146-1159. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

