Interleukin-10 Produced by Myeloid-Derived Suppressor Cells Provides Protection to Carbapenem-Resistant Klebsiella pneumoniae Sequence Type 258 by Enhancing Its Clearance in the Airways
- PMID: 30804104
- PMCID: PMC6479034
- DOI: 10.1128/IAI.00665-18
Interleukin-10 Produced by Myeloid-Derived Suppressor Cells Provides Protection to Carbapenem-Resistant Klebsiella pneumoniae Sequence Type 258 by Enhancing Its Clearance in the Airways
Abstract
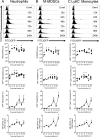
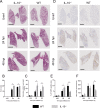
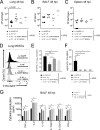
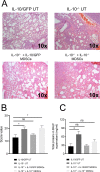
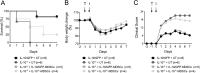
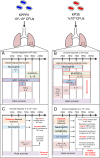
Carbapenem-resistant Klebsiella pneumoniae sequence type 258 (CRKP-ST258) can cause chronic infections in lungs and airways, with repeated episodes of bacteremia. In this report we addressed whether the recruitment of myeloid cells producing the anti-inflammatory cytokine interleukin-10 (IL-10) modulates the clearance of CKRP-ST258 in the lungs and establishes bacterial persistence. Our data demonstrate that during pneumonia caused by a clinical isolate of CRKP-ST258 (KP35) there is an early recruitment of monocyte-myeloid-derived suppressor cells (M-MDSCs) and neutrophils that actively produce IL-10. However, M-MDSCs were the cells that sustained the production of IL-10 over the time of infection evaluated. Using mice unable to produce IL-10 (IL-10-/-), we observed that the production of this cytokine during the infection caused by KP35 is important to control bacterial burden, to prevent lung damage, to modulate cytokine production, and to improve host survival. Importantly, intranasal transfer of bone marrow-derived M-MDSCs from mice able to produce IL-10 at 1 day prior to infection improved the ability of IL-10-/- mice to clear KP35 in the lungs, decreasing their mortality. Altogether, our data demonstrate that IL-10 produced by M-MDSCs is required for bacterial clearance, reduction of lung tissue damage, and host survival during KP35 pneumonia.
Keywords: Klebsiella pneumoniae ST258; interleukin-10; monocytic-myeloid-derived suppressor cells; neutrophils.
Copyright © 2019 American Society for Microbiology.
Figures



Similar articles
-
L-Arginine Enhances Intracellular Killing of Carbapenem-Resistant Klebsiella pneumoniae ST258 by Murine Neutrophils.Front Cell Infect Microbiol. 2020 Nov 13;10:571771. doi: 10.3389/fcimb.2020.571771. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33282749 Free PMC article.
-
Acquired resistance to innate immune clearance promotes Klebsiella pneumoniae ST258 pulmonary infection.JCI Insight. 2016 Oct 20;1(17):e89704. doi: 10.1172/jci.insight.89704. JCI Insight. 2016. PMID: 27777978 Free PMC article.
-
Novel, Broadly Reactive Anticapsular Antibodies against Carbapenem-Resistant Klebsiella pneumoniae Protect from Infection.mBio. 2018 Apr 3;9(2):e00091-18. doi: 10.1128/mBio.00091-18. mBio. 2018. PMID: 29615497 Free PMC article.
-
Antibody-Mediated Killing of Carbapenem-Resistant ST258 Klebsiella pneumoniae by Human Neutrophils.mBio. 2018 Mar 13;9(2):e00297-18. doi: 10.1128/mBio.00297-18. mBio. 2018. PMID: 29535199 Free PMC article.
-
Expanding the Current Knowledge About the Role of Interleukin-10 to Major Concerning Bacteria.Front Microbiol. 2018 Sep 18;9:2047. doi: 10.3389/fmicb.2018.02047. eCollection 2018. Front Microbiol. 2018. PMID: 30279680 Free PMC article. Review.
Cited by
-
An acquired acyltransferase promotes Klebsiella pneumoniae ST258 respiratory infection.Cell Rep. 2021 Jun 1;35(9):109196. doi: 10.1016/j.celrep.2021.109196. Cell Rep. 2021. PMID: 34077733 Free PMC article.
-
Advances in the study of myeloid-derived suppressor cells in infectious lung diseases.Front Immunol. 2023 Mar 29;14:1125737. doi: 10.3389/fimmu.2023.1125737. eCollection 2023. Front Immunol. 2023. PMID: 37063919 Free PMC article. Review.
-
Myeloid-derived suppressor cell inhibits T-cell-based defense against Klebsiella pneumoniae infection via IDO1 production.PLoS Pathog. 2025 Mar 17;21(3):e1012979. doi: 10.1371/journal.ppat.1012979. eCollection 2025 Mar. PLoS Pathog. 2025. PMID: 40096073 Free PMC article.
-
Distinctive features and prognostic utility of neutrophil in severe patients with Klebsiella pneumoniae infection.Front Cell Infect Microbiol. 2024 Sep 3;14:1406168. doi: 10.3389/fcimb.2024.1406168. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39290978 Free PMC article.
-
Finding Order in the Chaos: Outstanding Questions in Klebsiella pneumoniae Pathogenesis.Infect Immun. 2021 Mar 17;89(4):e00693-20. doi: 10.1128/IAI.00693-20. Print 2021 Mar 17. Infect Immun. 2021. PMID: 33558323 Free PMC article. Review.
References
-
- Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. 2013. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis 13:785–796. doi:10.1016/S1473-3099(13)70190-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

