Redox and pH gradients drive amino acid synthesis in iron oxyhydroxide mineral systems
- PMID: 30804197
- PMCID: PMC6421445
- DOI: 10.1073/pnas.1812098116
Redox and pH gradients drive amino acid synthesis in iron oxyhydroxide mineral systems
Abstract
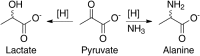
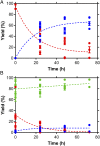
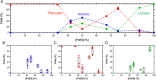
Iron oxyhydroxide minerals, known to be chemically reactive and significant for elemental cycling, are thought to have been abundant in early-Earth seawater, sediments, and hydrothermal systems. In the anoxic Fe2+-rich early oceans, these minerals would have been only partially oxidized and thus redox-active, perhaps able to promote prebiotic chemical reactions. We show that pyruvate, a simple organic molecule that can form in hydrothermal systems, can undergo reductive amination in the presence of mixed-valence iron oxyhydroxides to form the amino acid alanine, as well as the reduced product lactate. Furthermore, geochemical gradients of pH, redox, and temperature in iron oxyhydroxide systems affect product selectivity. The maximum yield of alanine was observed when the iron oxyhydroxide mineral contained 1:1 Fe(II):Fe(III), under alkaline conditions, and at moderately warm temperatures. These represent conditions that may be found, for example, in iron-containing sediments near an alkaline hydrothermal vent system. The partially oxidized state of the precipitate was significant in promoting amino acid formation: Purely ferrous hydroxides did not drive reductive amination but instead promoted pyruvate reduction to lactate, and ferric hydroxides did not result in any reaction. Prebiotic chemistry driven by redox-active iron hydroxide minerals on the early Earth would therefore be strongly affected by geochemical gradients of Eh, pH, and temperature, and liquid-phase products would be able to diffuse to other conditions within the sediment column to participate in further reactions.
Keywords: early Earth; gradients; hydrothermal vents; iron hydroxides; life emergence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Water near its Supercritical Point and at Alkaline pH for the Production of Ferric Oxides and Silicates in Anoxic Conditions. A New Hypothesis for the Synthesis of Minerals Observed in Banded Iron Formations and for the Related Geobiotropic Chemistry inside Fluid Inclusions.Orig Life Evol Biosph. 2018 Sep;48(3):289-320. doi: 10.1007/s11084-018-9560-y. Epub 2018 Aug 8. Orig Life Evol Biosph. 2018. PMID: 30091010 Free PMC article.
-
Energy sources for chemolithotrophs in an arsenic- and iron-rich shallow-sea hydrothermal system.Geobiology. 2011 Sep;9(5):436-45. doi: 10.1111/j.1472-4669.2011.00291.x. Geobiology. 2011. PMID: 21884364
-
Reactivity of Uranium and Ferrous Iron with Natural Iron Oxyhydroxides.Environ Sci Technol. 2015 Sep 1;49(17):10357-65. doi: 10.1021/acs.est.5b02645. Epub 2015 Aug 14. Environ Sci Technol. 2015. PMID: 26226398
-
Products of the iron cycle on the early Earth.Free Radic Biol Med. 2019 Aug 20;140:138-153. doi: 10.1016/j.freeradbiomed.2019.05.005. Epub 2019 May 6. Free Radic Biol Med. 2019. PMID: 31071438 Review.
-
Aqueous high-temperature and high-pressure organic geochemistry of hydrothermal vent systems.Geochim Cosmochim Acta. 1993;57:3231-43. doi: 10.1016/0016-7037(93)90536-6. Geochim Cosmochim Acta. 1993. PMID: 11539452 Review.
Cited by
-
Prebiotic synthesis of α-amino acids and orotate from α-ketoacids potentiates transition to extant metabolic pathways.Nat Chem. 2022 Oct;14(10):1142-1150. doi: 10.1038/s41557-022-00999-w. Epub 2022 Jul 28. Nat Chem. 2022. PMID: 35902742
-
Recent progress in primitive polyester synthesis and membraneless microdroplet assembly.Biophys Physicobiol. 2023 Feb 22;20(1):e200012. doi: 10.2142/biophysico.bppb-v20.0012. eCollection 2023. Biophys Physicobiol. 2023. PMID: 37234852 Free PMC article.
-
Unraveling abiotic organic synthesis pathways in the mafic crust of mid-ocean ridges.Proc Natl Acad Sci U S A. 2024 Oct 22;121(43):e2308684121. doi: 10.1073/pnas.2308684121. Epub 2024 Oct 10. Proc Natl Acad Sci U S A. 2024. PMID: 39388277 Free PMC article.
-
Nanoscale Anatomy of Iron-Silica Self-Organized Membranes: Implications for Prebiotic Chemistry.Angew Chem Int Ed Engl. 2021 Jan 18;60(3):1396-1402. doi: 10.1002/anie.202012059. Epub 2020 Nov 23. Angew Chem Int Ed Engl. 2021. PMID: 33022871 Free PMC article.
-
The Effects of Iron on In Silico Simulated Abiotic Reaction Networks.Molecules. 2022 Dec 13;27(24):8870. doi: 10.3390/molecules27248870. Molecules. 2022. PMID: 36558002 Free PMC article.
References
-
- MacLeod G, McKeown C, Hall AJ, Russell MJ. Hydrothermal and oceanic pH conditions of possible relevance to the origin of life. Orig Life Evol Biosph. 1994;24:19–41. - PubMed
-
- Halevy I, Bachan A. The geologic history of seawater pH. Science. 2017;355:1069–1071. - PubMed
-
- Tosca NJ, Guggenheim S, Pufahl PK. An authigenic origin for Precambrian greenalite: Implications for iron formation and the chemistry of ancient seawater. Geol Soc Am Bull. 2016;128:511–530.
-
- Halevy I, Alesker E, Schuster EM, Popovitz-Biro R, Feldman Y. A key role for green rust in the Precambrian oceans and the genesis of iron formations. Nat Geosci. 2017;10:135–139.
-
- Jolivet JP, Chanéac C, Tronc E. Iron oxide chemistry. From molecular clusters to extended solid networks. Chem Commun (Camb) 2004:481–487. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

