Resolution of human ribosomal DNA occurs in anaphase, dependent on tankyrase 1, condensin II, and topoisomerase IIα
- PMID: 30804226
- PMCID: PMC6411013
- DOI: 10.1101/gad.321836.118
Resolution of human ribosomal DNA occurs in anaphase, dependent on tankyrase 1, condensin II, and topoisomerase IIα
Abstract
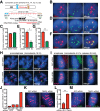
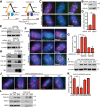
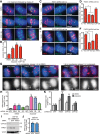
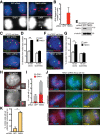
Formation of individualized sister chromatids is essential for their accurate segregation. In budding yeast, while most of the genome segregates at the metaphase to anaphase transition, resolution of the ribosomal DNA (rDNA) repeats is delayed. The timing and mechanism in human cells is unknown. Here we show that resolution of human rDNA occurs in anaphase after the bulk of the genome, dependent on tankyrase 1, condensin II, and topoisomerase IIα. Defective resolution leads to rDNA bridges, rDNA damage, and aneuploidy of an rDNA-containing acrocentric chromosome. Thus, temporal regulation of rDNA segregation is conserved between yeast and man and is essential for genome integrity.
Keywords: condensin; rDNA; tankyrase; topoisomerase IIa.
© 2019 Daniloski et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
