Downregulation of lysyl oxidase and lysyl oxidase-like protein 2 suppressed the migration and invasion of trophoblasts by activating the TGF-β/collagen pathway in preeclampsia
- PMID: 30804321
- PMCID: PMC6389995
- DOI: 10.1038/s12276-019-0211-9
Downregulation of lysyl oxidase and lysyl oxidase-like protein 2 suppressed the migration and invasion of trophoblasts by activating the TGF-β/collagen pathway in preeclampsia
Abstract
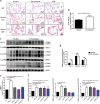
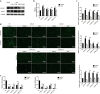
Preeclampsia is a pregnancy-specific disorder that is a major cause of maternal and fetal morbidity and mortality with a prevalence of 6-8% of pregnancies. Although impaired trophoblast invasion in early pregnancy is known to be closely associated with preeclampsia, the underlying mechanisms remain elusive. Here we revealed that lysyl oxidase (LOX) and LOX-like protein 2 (LOXL2) play a critical role in preeclampsia. Our results demonstrated that LOX and LOXL2 expression decreased in preeclamptic placentas. Moreover, knockdown of LOX or LOXL2 suppressed trophoblast cell migration and invasion. Mechanistically, collagen production was induced in LOX- or LOXL2-downregulated trophoblast cells through activation of the TGF-β1/Smad3 pathway. Notably, inhibition of the TGF-β1/Smad3 pathway could rescue the defects caused by LOX or LOXL2 knockdown, thereby underlining the significance of the TGF-β1/Smad3 pathway downstream of LOX and LOXL2 in trophoblast cells. Additionally, induced collagen production and activated TGF-β1/Smad3 were observed in clinical samples from preeclamptic placentas. Collectively, our study suggests that the downregulation of LOX and LOXL2 leading to reduced trophoblast cell migration and invasion through activation of the TGF-β1/Smad3/collagen pathway is relevant to preeclampsia. Thus, we proposed that LOX, LOXL2, and the TGF-β1/Smad3/collagen pathway can serve as potential markers and targets for clinical diagnosis and therapy for preeclampsia.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

