Extracellular signal-regulated kinase 5 increases radioresistance of lung cancer cells by enhancing the DNA damage response
- PMID: 30804322
- PMCID: PMC6389946
- DOI: 10.1038/s12276-019-0209-3
Extracellular signal-regulated kinase 5 increases radioresistance of lung cancer cells by enhancing the DNA damage response
Erratum in
-
Author Correction: Extracellular signal-regulated kinase 5 increases radioresistance of lung cancer cells by enhancing the DNA damage response.Exp Mol Med. 2023 Jun;55(6):1272-1274. doi: 10.1038/s12276-023-01026-9. Exp Mol Med. 2023. PMID: 37264203 Free PMC article. No abstract available.
Abstract
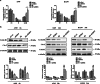
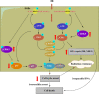
Radiotherapy is a frequent mode of cancer treatment, although the development of radioresistance limits its effectiveness. Extensive investigations indicate the diversity of the mechanisms underlying radioresistance. Here, we aimed to explore the effects of extracellular signal-regulated kinase 5 (ERK5) on lung cancer radioresistance and the associated mechanisms. Our data showed that ERK5 is activated during solid lung cancer development, and ectopic expression of ERK5 promoted cell proliferation and G2/M cell cycle transition. In addition, we found that ERK5 is a potential regulator of radiosensitivity in lung cancer cells. Mechanistic investigations revealed that ERK5 could trigger IR-induced activation of Chk1, which has been implicated in DNA repair and cell cycle arrest in response to DNA double-strand breaks (DSBs). Subsequently, ERK5 knockdown or pharmacological inhibition selectively inhibited colony formation of lung cancer cells and enhanced IR-induced G2/M arrest and apoptosis. In vivo, ERK5 knockdown strongly radiosensitized A549 and LLC tumor xenografts to inhibition, with a higher apoptotic response and reduced tumor neovascularization. Taken together, our data indicate that ERK5 is a novel potential target for the treatment of lung cancer, and its expression might be used as a biomarker to predict radiosensitivity in NSCLC patients.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

