Pancreatic acinar differentiation is guided by differential laminin deposition
- PMID: 30804366
- PMCID: PMC6389953
- DOI: 10.1038/s41598-019-39077-6
Pancreatic acinar differentiation is guided by differential laminin deposition
Abstract
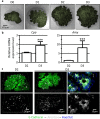
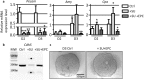
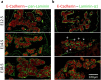
Endothelial cells play multiple roles during pancreas organogenesis. First, they are required to instruct endoderm-derived pancreatic progenitor cells to initiate branching morphogenesis. Later, blood vessels promote β-cell differentiation but also limit acinar development. In this work, we show how endothelial cells might signal to pancreatic progenitors and spatially regulate acinar differentiation. Using an ex vivo culture system of undifferentiated E12.5 pancreata, we demonstrate that embryonic endothelial progenitor cells and their conditioned medium prevent the expression of two members of the pro-acinar transcriptional PTF1L-complex. This effect is not mediated by SPARC, a protein abundantly released in the medium conditioned by endothelial progenitors. On the contrary, heterotrimeric laminin-α1β1γ1, also produced by endothelial progenitor cells, can repress acinar differentiation when used on its own on pancreatic explants. Lastly, we found that laminin-α1 is predominantly found in vivo around the pancreatic trunk cells, as compared to the tip cells, at E14.5. In conclusion, we propose that expression or deposition of laminin-α1β1γ1 around the trunk cells, where blood vessels are predominantly localized, prevent acinar differentiation of these cells. On the contrary, transient decreased expression or deposition of laminin-α1β1γ1 around the tip cells would allow PTF1L-complex formation and acinar differentiation.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Larsen, H. L. & Grapin-Botton, A. The molecular and morphogenetic basis of pancreas organogenesis. Seminars in Cell & Developmental Biology66, 51–68 (2017). - PubMed
-
- Zhou, Q. et al. A Multipotent Progenitor Domain Guides Pancreatic Organogenesis. Developmental Cell13(1), 103–114 (2007). - PubMed
-
- Kim SK, Hebrok M, Melton DA. Notochord to endoderm signaling is required for pancreas development. Development. 1997;124:4243–4252. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

