Extracellular matrix remodelling induced by alternating electrical and mechanical stimulations increases the contraction of engineered skeletal muscle tissues
- PMID: 30804393
- PMCID: PMC6389954
- DOI: 10.1038/s41598-019-39522-6
Extracellular matrix remodelling induced by alternating electrical and mechanical stimulations increases the contraction of engineered skeletal muscle tissues
Abstract
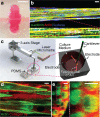
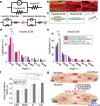
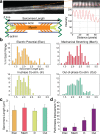
Engineered skeletal muscles are inferior to natural muscles in terms of contractile force, hampering their potential use in practical applications. One major limitation is that the extracellular matrix (ECM) not only impedes the contraction but also ineffectively transmits the forces generated by myotubes to the load. In the present study, ECM remodelling improves contractile force in a short time, and a coordinated, combined electrical and mechanical stimulation induces the desired ECM remodelling. Notably, the application of single and combined stimulations to the engineered muscles remodels the structure of their ECM networks, which determines the mechanical properties of the ECM. Myotubes in the tissues are connected in parallel and in series to the ECM. The stiffness of the parallel ECM must be low not to impede contraction, while the stiffness of the serial ECM must be high to transmit the forces to the load. Both the experimental results and the mechanistic model suggest that the combined stimulation through coordination reorients the ECM fibres in such a way that the parallel ECM stiffness is reduced, while the serial ECM stiffness is increased. In particular, 3 and 20 minutes of alternating electrical and mechanical stimulations increase the force by 18% and 31%, respectively.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Control of myotube contraction using electrical pulse stimulation for bio-actuator.J Artif Organs. 2009;12(2):131-7. doi: 10.1007/s10047-009-0457-4. Epub 2009 Jun 18. J Artif Organs. 2009. PMID: 19536631
-
Electrical stimulation increases hypertrophy and metabolic flux in tissue-engineered human skeletal muscle.Biomaterials. 2019 Apr;198:259-269. doi: 10.1016/j.biomaterials.2018.08.058. Epub 2018 Aug 31. Biomaterials. 2019. PMID: 30180985 Free PMC article.
-
Effect of cell-extracellular matrix interaction on myogenic characteristics and artificial skeletal muscle tissue.J Biosci Bioeng. 2020 Jul;130(1):98-105. doi: 10.1016/j.jbiosc.2020.02.008. Epub 2020 Apr 8. J Biosci Bioeng. 2020. PMID: 32278672
-
Evaluation systems of generated forces of skeletal muscle cell-based bio-actuators.J Biosci Bioeng. 2013 Feb;115(2):115-21. doi: 10.1016/j.jbiosc.2012.08.024. Epub 2012 Sep 29. J Biosci Bioeng. 2013. PMID: 23026451 Review.
-
Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading.Physiol Rev. 2004 Apr;84(2):649-98. doi: 10.1152/physrev.00031.2003. Physiol Rev. 2004. PMID: 15044685 Review.
Cited by
-
Multicellular muscle-tendon bioprinting of mechanically optimized musculoskeletal bioactuators with enhanced force transmission.Sci Adv. 2025 Jul 18;11(29):eadv2628. doi: 10.1126/sciadv.adv2628. Epub 2025 Jul 16. Sci Adv. 2025. PMID: 40668913 Free PMC article.
-
Tissue-in-a-Tube: three-dimensional in vitro tissue constructs with integrated multimodal environmental stimulation.Mater Today Bio. 2020 Jul 28;7:100070. doi: 10.1016/j.mtbio.2020.100070. eCollection 2020 Jun. Mater Today Bio. 2020. PMID: 32875285 Free PMC article.
-
CCN2 participates in overload-induced skeletal muscle hypertrophy.Matrix Biol. 2022 Feb;106:1-11. doi: 10.1016/j.matbio.2022.01.003. Epub 2022 Jan 16. Matrix Biol. 2022. PMID: 35045313 Free PMC article.
-
Tri-culture of spatially organizing human skeletal muscle cells, endothelial cells, and fibroblasts enhances contractile force and vascular perfusion of skeletal muscle tissues.FASEB J. 2022 Aug;36(8):e22453. doi: 10.1096/fj.202200500R. FASEB J. 2022. PMID: 35838893 Free PMC article.
-
Skeletal muscle-specific overexpression of miR-486 limits mammary tumor-induced skeletal muscle functional limitations.Mol Ther Nucleic Acids. 2022 Mar 16;28:231-248. doi: 10.1016/j.omtn.2022.03.009. eCollection 2022 Jun 14. Mol Ther Nucleic Acids. 2022. PMID: 35402076 Free PMC article.
References
-
- Corona BT, Ward CL, Baker HB, Walters TJ, Christ GJ. Implantation of in vitro tissue engineered muscle repair constructs and bladder acellular matrices partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss injury. Tissue Eng. Part A. 2014;20:705–715. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

