Biomolecular Interaction, Anti-Cancer and Anti-Angiogenic Properties of Cobalt(III) Schiff Base Complexes
- PMID: 30804454
- PMCID: PMC6389928
- DOI: 10.1038/s41598-019-39179-1
Biomolecular Interaction, Anti-Cancer and Anti-Angiogenic Properties of Cobalt(III) Schiff Base Complexes
Abstract
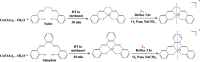
Two cobalt(III) Schiff base complexes, trans-[Co(salen)(DA)2](ClO4) (1) and trans-[Co(salophen)(DA)2](ClO4) (2) (where salen: N,N'-bis(salicylidene)ethylenediamine, salopen: N,N'-bis(salicylidene)-1,2-phenylenediamine, DA: dodecylamine) were synthesised and characterised using various spectroscopic and analytical techniques. The binding affinity of both the complexes with CT-DNA was explored adopting UV-visible, fluorescence, circular dichroism spectroscopy and cyclic voltammetry techniques. The results revealed that both the complexes interacted with DNA via intercalation as well as notable groove binding. Protein (BSA) binding ability of these complexes was investigated by absorption and emission spectroscopy which indicate that these complexes engage in strong hydrophobic interaction with BSA. The mode of interaction between these complexes and CT-DNA/BSA was studied by molecular docking analysis. The in vitro cytotoxic property of the complexes was evaluated in A549 (human small cell lung carcinoma) and VERO (African green monkey kidney cells). The results revealed that the complexes affect viability of the cells. AO and EB staining and cell cycle analysis revealed that the mode of cell death is apoptosis. Both the complexes showed profound inhibition of angiogenesis as revealed in in-vivo chicken chorioallantoic membrane (CAM) assay. Of the two complexes, the complex 2 proved to be much more efficient in affecting the viability of lung cancer cells than complex 1. These results indicate that the cobalt(III) Schiff base complexes in this study can be potentially used for cancer chemotherapy and as inhibitor of angiogenesis, in general, and lung cancer in particular, for which there is need for substantiation at the level of signalling mechanisms and gene expressions.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

