Surface chemical defence of the eelgrass Zostera marina against microbial foulers
- PMID: 30804483
- PMCID: PMC6389981
- DOI: 10.1038/s41598-019-39212-3
Surface chemical defence of the eelgrass Zostera marina against microbial foulers
Abstract
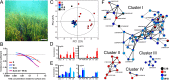
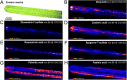
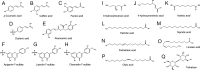
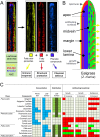
Plants rely on both mechanical and chemical defence mechanisms to protect their surfaces against microorganisms. The recently completed genome of the eelgrass Zostera marina, a marine angiosperm with fundamental importance for coastal ecosystems, showed that its re-adaptation from land to the sea has led to the loss of essential genes (for chemical communication and defence) and structural features (stomata and thick cuticle) that are typical of terrestrial plants. This study was designed to understand the molecular nature of surface protection and fouling-control strategy of eelgrass against marine epiphytic yeasts. Different surface extraction methods and comparative metabolomics by tandem mass spectrometry (LC-MS/MS) were used for targeted and untargeted identification of the metabolite profiles of the leaf surface and the whole tissue extracts. Desorption electrospray ionization-imaging mass spectrometry (DESI-IMS) coupled with traditional bioassays revealed, for the first time, the unique spatial distribution of the eelgrass surface-associated phenolics and fatty acids, as well as their differential bioactivity against the growth and settlement of epiphytic yeasts. This study provides insights into the complex chemical defence system of the eelgrass leaf surface. It suggests that surface-associated metabolites modulate biotic interactions and provide chemical defence and structural protection to eelgrass in its marine environment.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Frederiksen MS, Glud RN. Oxygen dynamics in the rhizosphere of Zostera marina: A two-dimensional planar optode study. Limnol Oceanogr. 2006;51:1072–1083. doi: 10.4319/lo.2006.51.2.1072. - DOI
-
- Gustafsson C, Boström C. Biodiversity influences ecosystem functioning in aquatic angiosperm communities. Oikos. 2011;120:1037–1046. doi: 10.1111/j.1600-0706.2010.19008.x. - DOI
-
- Hume AC, Berg P, McGlathery KJ. Dissolved oxygen fluxes and ecosystem metabolism in an eelgrass (Zostera marina) meadow measured with the eddy correlation technique. Limnol Oceanogr. 2011;56:86–96. doi: 10.4319/lo.2011.56.1.0086. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

