Bioinspired neuron-like electronics
- PMID: 30804509
- PMCID: PMC6474791
- DOI: 10.1038/s41563-019-0292-9
Bioinspired neuron-like electronics
Abstract
As an important application of functional biomaterials, neural probes have contributed substantially to studying the brain. Bioinspired and biomimetic strategies have begun to be applied to the development of neural probes, although these and previous generations of probes have had structural and mechanical dissimilarities from their neuron targets that lead to neuronal loss, neuroinflammatory responses and measurement instabilities. Here, we present a bioinspired design for neural probes-neuron-like electronics (NeuE)-where the key building blocks mimic the subcellular structural features and mechanical properties of neurons. Full three-dimensional mapping of implanted NeuE-brain interfaces highlights the structural indistinguishability and intimate interpenetration of NeuE and neurons. Time-dependent histology and electrophysiology studies further reveal a structurally and functionally stable interface with the neuronal and glial networks shortly following implantation, thus opening opportunities for next-generation brain-machine interfaces. Finally, the NeuE subcellular structural features are shown to facilitate migration of endogenous neural progenitor cells, thus holding promise as an electrically active platform for transplantation-free regenerative medicine.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures
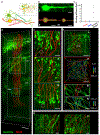


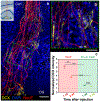
Comment in
-
Neuron-like neural probes.Nat Mater. 2019 May;18(5):429-431. doi: 10.1038/s41563-019-0312-9. Nat Mater. 2019. PMID: 30804508 Free PMC article.
References
-
- Ma X et al. Tuning crystallization pathways through sequence engineering of biomimetic polymers. Nat. Mater 16, 767–774 (2017). - PubMed
-
- Fratzl P, Kolednik O, Fischer FD & Dean MN The mechanics of tessellations – bioinspired strategies for fracture resistance. Chem. Soc. Rev 45, 252–267 (2016). - PubMed
-
- Sadtler K et al. Design, clinical translation and immunological response of biomaterials in regenerative medicine. Nat. Rev. Mater 1, 16040 (2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

