Innate Lymphoid Cells: A Link between the Nervous System and Microbiota in Intestinal Networks
- PMID: 30804706
- PMCID: PMC6360575
- DOI: 10.1155/2019/1978094
Innate Lymphoid Cells: A Link between the Nervous System and Microbiota in Intestinal Networks
Abstract
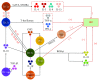
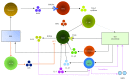
Innate lymphoid cells (ILCs) are a novel family of innate immune cells that act as key coordinators of intestinal mucosal surface immune defense and are essential for maintaining intestinal homeostasis and barrier integrity by responding to locally produced effector cytokines or direct recognition of exogenous or endogenous danger patterns. ILCs are also involved in the pathogenesis of inflammatory bowel disease (IBD). Many studies have demonstrated the occurrence of crosstalk between ILCs and intestinal microbiota, and ILCs have recently been shown to be connected to the enteric nervous system (ENS). Thus, ILCs may act as a key link between the nervous system and microbiota in intestinal networks. In this review, we briefly summarize the role of the ILCs in the intestinal tract (particularly in the context of IBD) and discuss the relationship between ILCs and the microbiota/ENS.
Figures
Similar articles
-
Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease.Int J Mol Sci. 2021 Jul 16;22(14):7618. doi: 10.3390/ijms22147618. Int J Mol Sci. 2021. PMID: 34299236 Free PMC article. Review.
-
Cross-talk between type 3 innate lymphoid cells and the gut microbiota in inflammatory bowel disease.Curr Opin Gastroenterol. 2015 Nov;31(6):449-55. doi: 10.1097/MOG.0000000000000217. Curr Opin Gastroenterol. 2015. PMID: 26398682 Free PMC article. Review.
-
Innate Lymphoid Cells in Inflammatory Bowel Disease.Cells. 2025 Jun 2;14(11):825. doi: 10.3390/cells14110825. Cells. 2025. PMID: 40498001 Free PMC article. Review.
-
Regulation of intestinal health and disease by innate lymphoid cells.Int Immunol. 2014 Sep;26(9):501-7. doi: 10.1093/intimm/dxu052. Epub 2014 May 12. Int Immunol. 2014. PMID: 24821261 Free PMC article.
-
Group 3 ILCs: Peacekeepers or Troublemakers? What's Your Gut Telling You?!Front Immunol. 2019 Apr 5;10:676. doi: 10.3389/fimmu.2019.00676. eCollection 2019. Front Immunol. 2019. PMID: 31024537 Free PMC article. Review.
Cited by
-
Mucosal Immunity and the Gut-Microbiota-Brain-Axis in Neuroimmune Disease.Int J Mol Sci. 2022 Nov 1;23(21):13328. doi: 10.3390/ijms232113328. Int J Mol Sci. 2022. PMID: 36362150 Free PMC article. Review.
-
Innate Lymphoid Cells and Natural Killer Cells in Bacterial Infections: Function, Dysregulation, and Therapeutic Targets.Front Cell Infect Microbiol. 2021 Nov 5;11:733564. doi: 10.3389/fcimb.2021.733564. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34804991 Free PMC article. Review.
-
Reversal of the detrimental effects of social isolation on ischemic cerebral injury and stroke-associated pneumonia by inhibiting small intestinal γδ T-cell migration into the brain and lung.J Cereb Blood Flow Metab. 2023 Aug;43(8):1267-1284. doi: 10.1177/0271678X231167946. Epub 2023 Apr 5. J Cereb Blood Flow Metab. 2023. PMID: 37017434 Free PMC article.
-
The Role of Gut Microbiota Biomodulators on Mucosal Immunity and Intestinal Inflammation.Cells. 2020 May 16;9(5):1234. doi: 10.3390/cells9051234. Cells. 2020. PMID: 32429359 Free PMC article. Review.
-
Alterations in Gut Microbiota and Upregulations of VPAC2 and Intestinal Tight Junctions Correlate with Anti-Inflammatory Effects of Electroacupuncture in Colitis Mice with Sleep Fragmentation.Biology (Basel). 2022 Jun 25;11(7):962. doi: 10.3390/biology11070962. Biology (Basel). 2022. PMID: 36101343 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

